Calculating Moles of an Element
Introduction to Moles
Focus: Calculating the number of moles of an element.
Topic: Higher tier chemistry.
Goal: Understand how to use mathematics in chemistry to perform calculations.
Key Concepts
Relative Atomic Mass
Definition: Mass of a single atom of an element.
Calculation: Average masses of isotopes weighted for abundance.
Units: Relative atomic mass has no units.
Examples:
Carbon (C): 12
Oxygen (O): 16
The Mole
Definition: A mole refers to a large number of atoms, specifically 6.022 x 10²³ atoms (not fully discussed in this transcript).
Practical Use: Allows chemists to calculate the number of atoms in a chemical reaction.
Relationship: The relative atomic mass of any element in grams equals one mole of that element.
Example: 12g of Carbon = 1 mole of Carbon atoms; 16g of Oxygen = 1 mole of Oxygen atoms:

The relative atomic mass of carbon is 12:
If I take 12 grams of carbon, then I've got one mole of carbon atoms. In other words, this number of carbon atoms. Looking at oxygen again, we've seen that the relative atomic mass of oxygen is 16. If we take 16 grams of oxygen, then we've got one mole of oxygen atoms. In other words, this number of oxygen atoms.
If we take the relative atomic mass of any element in grams, then we've got one mole of atoms of that element.
Calculating Moles
If we know the mass of an element that we're using in a chemical reaction, then we can calculate the number of moles using this equation:
Formula: Number of moles = Mass (g) / Relative atomic mass
Examples of Mole Calculations
Example 1: Magnesium
Given: 48g of magnesium
Relative atomic mass: 24
Calculation: 48g / 24 = 2 moles of magnesium.
Example 2: Calcium
Given: 120g of calcium
Relative atomic mass: 40
Calculation: 120g / 40 = 3 moles of calcium.
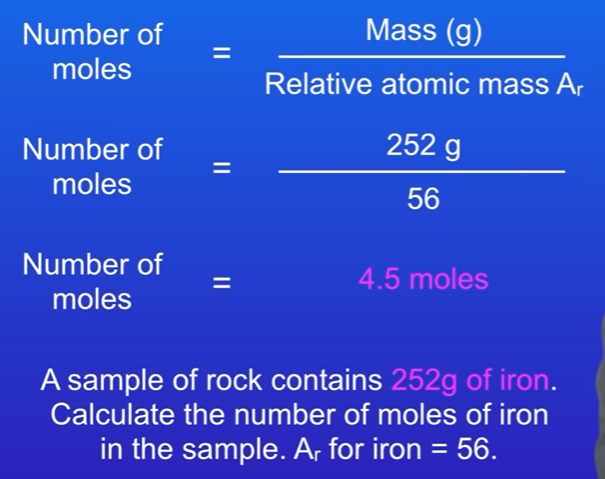
Example 3: Iron
Given: 252g of iron
Relative atomic mass: 56
Calculation: 252g / 56 = 4.5 moles of iron.
Example 4: Sulfur
Given: 4,064g of sulfur
Relative atomic mass: 32
Calculation: 4,064g / 32 = 127 moles of sulfur.
Conclusion
Summary: Mole calculations are manageable and essential for chemistry.
Additional Resources: More examples can be found in the revision workbook.