All units
UNIT 1
Naturally occurring elements in the body
Major Elements = 96%
Oxygen 65%
Carbon 18.5%
Hydrogen 9.5%
Nitrogen 3.3%
Essential Elements = 4%
Calcium
Phosphorus
Potassium
Sulfur
Sodium
Chlorine
Magnesium
Trace Elements = 0.01%
Copper: hair and skin
Zinc: enzyme function
Iodine: salt good for thyroid
Iron: needed in transport O2 in blood
Cofactor: Allows enzymes to function
Poly unsaturated: plant fat with many double bonds
Monounsaturated: plant fat with one double bond
Dietary Fiber: need 6 g to feel full
Protein-rich: 5g-6g
BHT: to pressure food and persevere food. It gives things shelf life
Things to check on the nutrition label
Serving Size
Calories
Trans fat; toxic & manmade
Radioisotope: an isotope of an element that has an unstable nuclei
Radioactive decay: the process by which an unstable nucleus rearranges itself
Electronegativity: Tendency of an atom to attract electrons
Partial charges: do not let electricity to pass through
Ionic bonds: Transfer of electrons between two unstable atoms
pH effect on the environment:
Acid Rain
Coral reef
Carbon
Element of life
Bonds:
Does not want to have charge; it only wants covalent bonds.
The double bond allows for fixed position
The single bond allows for rotation
Hydrocarbon properties:
Length
Double bonds
Branching
Rings
Methane: CH4
Ethane: C2H6
Ethene: C2H4
Most common Functional Groups attached to carbon
Methyl: R-CH3
UNIT3
Biomolecules (macromolecules)
Carbohydrates *
Lipids
Proteins *
Nucleic Acids *
* Polymers: chemicals made up of many repeating units called monomers
Dehydration RXN (condensation)
Short polymer with and H and monomer with OH connect to form a polymer with H2O
Hydrolysis RXN
Large polymer with H2O breaks into short polymer with and H and monomer with OH
Enzymes: proteins that allow rxns to take place
Hydrolytic enzymes: enzymes that use water to break down polymers
Carbohydrates
Empirical formula: CH2O
Monomers of carbohydrates are called Monosaccharides.
Functions:
Provide energy for all organisms
Structure of plant fungi and bacteria
Communication for cells
Glucose
Structure of Glucose
If a B glu and B glu connect one of them has to flip.
Polysaccharide
Starch
Very long chains of A-Glu (1000>)
Amylose - short chain
Amylopein - branch
Storage of energy for pants
Glycogen
Very long branched chains of A-Glu (1000>)
Highly branched
Storage of energy in liver/muscle cells
Chitin (CH2ON)
Exoskeleton of insects
Makes up cell wall of fungi
Straight chain
Cellulose
Only made up of B-Glu
Makes up the cell wall for plants
Made up of straight chains
What makes cells strong and rigid?
H bonds between cellulose molecules
Microfibril: a group of cellulose molecules holding onto each other
When many are stacked, they form the cell wall
Adaptive tissue: fat tissue
Our body stores sugar as glucose and excess sugar is stored as body fat
Proteins
All functions in the body are carried out by proteins
Protein could be used as energy once all other sources run out, this state is called starvation
Components
20 different types of amino acids
Nonpolar (hydrophobic)
Polar (hydrophilic)
Electrically charged (ionic)
Examples:
Nonpolar: Methionine
Polar: All polar amino have O or N at it’s end
Except for Cysteine, helps for 3D structure of proteins
The basic structure of an amino acid:
Elements found in all proteins
Carbon
Oxygen
Nitrogen
Hydrogen
Sulfur
Structure level of proteins
Primary structure 1°
Tells the number of amino acids
Types of amino acids
Sequence of amino acids
Secondary structure 2°
Folding of the 1° structure using H-Bonds
α Helix (spiral)
Exp: collagen, holds cells together
β pleated sheets
Exp: keratin, for hair and nails
Fibrous Protein
Proteins that have up to the 2° structure and they are insoluble in water, nonpolar, hydrophobic
Tertiary structure 3°
Further folding and twisting of previous structures by using Intra molecular forces such as:
H Bonds
LDF
Ionic bonds
Dip Dip
Disulfide bridges
Exp: hemoglobin or insulin
Globalor proteins:
They have 3° structure or higher, and they are soluble in water
Quateunary structure 4°
Found in proteins that have 2 or more polypeptide chains held together by intra-MF
Exp:
Hemoglobin: 4 polypeptide
Indulin: 2 polypeptide
Denaturation
loss of protein structure leading to loss of functions
Caused by:
pH
Temperature
Pressure
Salt → have charged when dissolved in H2O, disrupts ionic bonds
↑ maybe reversible or irreversible
Structure of protein
Defence → antibodies → protects from bacteria and viruses
Enzymes → Alpha amylase → breaks down starch
Structure → collagen → molecules have 2° fibers have 4°
Communication → insulin (dec glucose) glucagon (inc glucose)
Storage → ferritin → stores iron
Transport→ calcium pump → moves Ca^2+ across membrane
HDL → lipids around body
Hemoglobin → transports O2 in blood
Contractile proteins → myosin and actin → contract & move muscles
Receptors → insulin receptor → detect hormones
Nucleic Acid
Central dogma: All life depends on DNA
DNA:
deoxyribonucleic acid
Double helix
It has 2 strands with H-Bonds in the middle.
RNA: ribonucleic acid
1 strange with different types
mRNA (message) - carries the info from the nucleus to the cytoplasm
tRNA (transfer) - brings the correct amino acid during protein synthesis
rRNA (ribosomal) - needed in structure for ribosomes
call structure that builds proteins
miRNA (micro) - regulates gene expression
DNA → RNA → PROTEINS
from DNA to RNA: Transcription
from RNA to protein: Translation
Transcription:
It happens in the nucleus
Type of chemical remains the same but the structure changes
Translation:
Happens in the cytoplasm
Type of chemical changes and so does the structure
Monomers of nucleic acids: nucleotides
Made up of:
Nitrogenous base ↓H⁺ attached to C1
Pentose sugar C5H10O5
Phosphate group Always attached to C5
Lipids
Fats
Animal sources
Solid at room temp
Saturated
Oils
Plant source
Liquid at room temp
Unsaturated
Basic functions of lipids
Energy storage
Insulation
Cell membranes (make new cells)
Communication for hormones
Defence
Triglycerides
Used for energy storage
Made up of
1 glycerol
3 fatty acids
Fatty: hydrocarbon
Acid: carboxyl
Structure
Esterification: the process of creating an ester bond rxn between alcohol and fatty acid
Types of triglycerides
Saturated triglycerides
All three fatty acids must have single bonds ONLY
Monounsaturated
One and only one
double bond must be present, causing one of the fatty acids to move downwards, allowing it to be liquid at room temp. Because it leaves space for it to move and become a liquid
Polyunsaturated
Has two or more double bonds present. It can be on the same fatty acid
Vitamins
V. are special chemicals needed for proper metabolism
Nonpolar are stored in adopost tissue
Polar ones cannot be stored
Retinol: V A1 alcohol nonpolar
Retinal: V A aldehyde nonpolar
VD: nonpolar cholesterol-based forms on skin
Phospholipids
Create the cell membranes.
Made up of:
Glycerol
2 fatty acids
Phosphate group
Steroids
Creation of hormones
Membrane stability (flexibility)
Regulates fluidity
More cholesterol more rigid
Components
Cholesterol
Cholesterol Rings
Waxes
For defence
Made up of:
Long chained alcohol
Long chained fatty acid
UNIT 4
Microscopes
Light
Uses light as an energy source
Mirrors direct the light to your eyes
Lenses are used to enlarge and focus image
Electron
Uses electricity as energy source
Magnets direct flow of electrons for viewing
Scanning
e- reflect off the specimen for 3d viewing
Transmission
e- flow through providing an internal structure
Structure of Cell membrane
Fluid mosaic model
Fluid
Phospholipids: create flexible bilayers act as water barrier
Cholesterol: regulates the fluidity of the cell membrane
Mosaic
Proteins: carry out various functions
Transport proteins move substances from one side of the membrane to another.
Why are cells small in size
Prokaryotic Cell
Cytoplasm: Gel-like fluid (cytosol) that contains chemicals (proteins) and cell structures (ribosomes) inner region of the cell contained by cell membrane
Bacterial chromosome: circular DNA that contains genetic information
Nucleoiod: region of the cytoplasm where the bacterial chromosome is located
Ribsomoes: Proton synthesis, creation of proteins for the cell
Plasmid: extra small pieces of DNA that provide superpowers to cells
Antibiotic resistance: cant be fought off by antibodies & neither will its offsprings
Motility: mobility by itself & spreads easily
Production of a capsule: proteins itself & moves around & sticks to surfaces
Fertility: ability to reproduce sexually, creation of new bacteria
Pathogenic: ability to cause infection or disease
Cell membrane: made up of phospholipids, cholesterol proteins & carbohydrates, it creates the boundaries of the cell it acts as a barrier controlling what comes in & out
Cell well: made up of a mixture of carbohydrates and proteins called peptidoglycan provies structure & shape
Capsule: may or may not be present, attaches to surfaces to induce infections, strong enough to resist digestion by white blood cells
Flagella: made up of protons, extending from the cell membrane, allow cells to move, moves by rotation.
Fimbriae: made up of protons that extend from the cell membrane for attachment ot surfaces.
Extracellular fluid: fluid on the outside of the cell
Eukaryotic Cells
Have compartmentalisation while prokaryotic don’t
The process of creating compartments closed off within the cell
Vacuole
Large vesicles with specialised functions
Food vacuole
Part of phagocytosis brings in large substances
Central vacuole
Found in plants; usually the largest structure
Stores water to create ‘pressure’ that helps with structure & shape of the cell & plant
Stores chemicals
Stores waste
Contractile vacuole
Found in freshwater protists; like the paramecium
Removes excess water from the cell
Water always moves to regions that have more solutes.
Paramecium
Water rushes in to fill up the vacuole when it reaches a certain point it opens a trap dopr and push it out to maintain a certain pressure
Endomembranal System
Nucleus
Controls the function of the cell & stores genetic material
Nucleolus: stains deeply create rRNA & tRNA
Chromatin: unwinded DNA wrapped around proteins
Nucleoplasm: the cytoplasm of a nucleus
Inner nuclear membrane: inner wall
Outer nuclear membrane: outer wall
Nuclear pores: openings between the membranes
Proton complex control the opening and closing of the pores
Ribosomes: made up of proteins & rRNA on outer membrane
Nuclear envelope: where the nucleus is held
Ribosomes
Proteins synthesis
Made up of two parts; large subunit and small subunit
Made with proteins & rRNA
Bound ribosomes: attached to the membrane
Free ribosomes: located in the cytoplasm
Bound ribosome creates proteins for:
Organisms they are attached to
Other organelles
Cell membrane
Exporting out the cell
Endoplasmic reticulum (ER)
Endo: in
Plasmic: thick fluid
Reticulum: network
Smooth ER
Tubular network of membrane
Liquid synthesis stores Ca^2+ detoxification of harmful chemicals
Rough ER
Flattened sack-like membrane covered with in bound ribosomes for protein synthesis
Functions
Bound ribosomes release the polypeptide chain into the lumen of the RER
Lumen: space contained within a structure
Polypeptide chain twists & folds into 2 or 3-degree structure % sugar chains are attached to create glycoprotein.
Sugar chains act as tags for sorting & delivering.
Glycoproteins are sorted into the ends of the RER for delivery when the transport vesicle is created by building
Trans vesicle
Budding: a process that creates vesicles by pinching off segments from a membrane
Newly created transport vehicle packages with protons is delivered to the next destination.
Vesicles: membrane-bound structures that have a spherical appearance and store or transport substances
Big ones are called vacuoles
Golgi Apparatus
Functions
Receives chemicals from other organelles
Modifies the chemicals
Sorts & packages the chemicals
Ships the chemicals to their final destination
Possible destination
Other organelles
Cell membrane
Export out of the cell
They become new organelles
Exp: Lysosome
Lysosome
Specialised vesicle filled with hydrolytic enzymes
Breaking down… using water
Protons
Carbohydrates
Lipids
Nucleic acid
Major functions
Phagocytosis
A cell process that involves lysosomes which breaks down large structures
Autophagy
A cell process where the cell digests…
Old worn-out organelles
Damages organelles
Uneeded organelles
End of endomembranal system
Energy organelles
Mitochondrion
Generates energy in the form of ATP
Found in all eukaryotic cell
Chloroplast
Harvest light to produce food by photosynthesis found in plants & algae
Chlorophyll is the chemical that absorbs light to provide energy to the chloroplast
Cytoskeleton
Skeleton of the cell located in the cytosol
Cytoplasm = cytosol + organelles
Components
Flagells & cilia
Flagella
Few and very long
Sperm cell
Cilia
Many & very short
Inside the throat
Flagells & cilia have the microtubles in a 9 (doubles) and 2 (singles) arrangement
Extra Cellular Matrix
Located on the outside of the cell made up of a network of proteins and carbohydrates found in the extracellular fluid & attached to the cell membrane
Fibronectin: holds cell matric to the membrane.
Functions
Holds cells together, creating tissues using collagen
Communication between cells
Identification of self using immunoglobulins (glycoproteins)
Cell junction
Attach cells together that are adjacent to each other.
Functions:
Tight junction
Holds cells tightly, preventing any substance from passing between them
Important for the skin & intestine
Made up of intermediate filaments (fibrous)
Desmosome (anchoring J.)
Holds cells together in place but not tightly
Important for lung cells & cells in capillaries
Made up of intermediate filimates
Gap junction
Membrane protein (globular) that connects two adjacent cells to create tunnels
which allows the cytoplasm of one cell to mix with the cytoplasm of another
Such as cardiac muscle
Cell Wall Plants
Made up of cellulose (B Glu)
Provides the shape of a structure of a plant cell
Resits pressure created by cells to become rigid
All plants have a primary cell wall, but some woody plants will also have a secondary cell wall
Cell walls are always built on the outside of the cell membrane.
Plasmodesmata
Plant cell junctions create opening which connect the cytoplasm of adjacent cells
Permit rapid movement & communication between plant cells
Peroxisomes
Specialised vesicles filled with hydrogen peroxide (H2O2)
Mainly found in plants (found only in our liver)
Oxidation of amino acids & fatty acids
Detoxify poison
* not produced by Golgi apparatus
Anatomy of a microscope
Handling
Hold with 2 hands
Don’t slide the microscope
Rotate the head if someone wants to see
Parts
Eyepiece (ocular lens)
Magnifies specimen 10x
Diopter adjustment
Zooms in & out
Head
Tube w/ mirrors direct light to the eye
Nose piece
Changes objective lens
Objective lens
Scanning objective lens (4x)
Low-power objective lens (10x)
High-power objective lens (40x)
Oil immersion lens (100x)
Arm
Holds upper structure
Course adjustment
Moves stage up & down quickly
Fine adjustment
Moves stage slightly up & down
Stage
Where you place your specimen/ must be over the hole
Stage clip
Holds specimen in place
Stage control
Moves stage towards or away
Moves stage left or right
Condensor
Controls how much light is coming in (pupil)
Brightness adjustment
Dimmer switch
Illumination
Light source
Light switch
Base
Supports the microscope, keeps it sturdy
How to draw your specimen
Use a pencil & unlined paper.
Take up most of the paper
Draw on the left side
Boundary structures of the specimen is more important than the secondary
Stipple method to show dark regions
Draw clear, continuous lines, no sketches
Draw part by part → look & draw
Label parts you can identify; lines need to be drawn by a ruler
Do not cross label lines; must touch what they point to
No arrowheads
Include descriptors
Include magnification used
Possible specimen descriptors
Cross section
Longitudinal section
Dry mount
Water mount
Stained
Include the type of magnification bottom right
UNIT 5
Membrane Proteins
Transport proteins
May or may not use ATP for energy to move chemicals in or out of the cell
Signal transduction pathway: involves the use of signalling molecules (either protons or lipids that carry messages for the cell; maybe protein an exp is insulin) Which carry the message to target cells (cells that are able to receive the signalling molecules because they have the appropriate receptor) The receptor (protein that accepts the signalling molecule) locate don the target cells will then release a series of chemicals into the cytoplasm to carry the message to the nucleus.
Extracellular matrix
Cytoplasm
Intercellular junctions
Cell-cell recognition
Relies on glycoproteins, macromolecules made up of proteins and carbohydrates. To helps cells in multi-cellular organisms identify each other as self
Enzymatic activity
Enzymes which are proteins that speed up or facilitate a reaction, can be located in the cell membrane.
Molecules are able to move because of their own kinetic molecular energy (KME)
What causes molecules to move the way they do?
Transport proteins
Channel & carrier → passive
Pump → active
Uniporters
Move chemicals in one direction
Either in or out of a cell
Exp: aquaporin
Ssymporters
Move 2 different chemicals in one direction
Both either into the cell or out
Exp: glucose/Na* contransport
Antiporters
Move different chemicals in the opposite direction
One goes into the cell and the other goes out
Exp: Na* / K* pump
Passive Transport
Diffusion
Diffusion
Movement of the substances from high [ ] to low [ ] movement of their substances down their concentrated gradient
When molecules move from a concentration to a less concentrated area
Electrical charge when molecules always move to balance charges
Osmosis
Selectively permeable membrane
Controls what goes from one side to another
Through this membrane, water will move from an area of high concentration to an area of low concentration.
Type of solution when comparing
Isotonic
A solution with a similar concentration of solutes
Hypotonic
A solution with a lower concentration of solutes than the ones around it
Hypertonic
A solution with a higher concentration of solutes than the ones around it
Animal Cells
Hypotonic
Inside is hyper so H2O rushes in and it bursts, becoming Lysed
Isotonic *
Rate of water going in matches the rate of water going out (ideal)
Hypertonic
Inside is hypo so H2O leaves making the cell shrivelled
Plant Cells
Hypotonic *
Water enters the cell while extra H2O enters the central vacuole creating pressure on the cell wall, making it turgid (ideal)
Isotonic
The rate of water going in equals the rate of water going out; leaves will be droopy because of the lack of pressure on the cell wall. The cell becomes Flaccid or deflated; it can survive, but it is ineffective.
Hypertonic
The water leaves the cell, causing the cell membrane to get ripped off the cell wall, called plasmolysed.
Facilitated diffusion
Movement of soluted from high [solute] to low [solute] with the help of transport proteins
Transport proteins: Allow cell membrane to be selective
Channel Proteins
Create openings or tunnels for specific solutes
Maybe gated
The nature & size of the tunnel allow what goes through
Exp: Na* channel / Aquaporin (allows water in)
Carrier Proteins
Change shape to carry specific solutes across the cell membrane
How it works
When it is in the normal conformation, it will face either the outside or inside of the cell
Specific solute enters the binding sight, which causes the carrier protein to flip counter motion and face the opposite side
Binding sight is the region of a protein where a substrate attaches
In the flipped counter motion the solute is repelled out of the character protein into the new region
The carrier proteins will return back to its normal conformation because no solute is in the binding site
Active Transport
Cells must supply energy in the form of ATP to move solutes form low [solute] to high [solute], working against the concentration gradient
Exp: sodium-potassium pump
Two forms of active transport
Primary active transport
A pump uses ATP to move chemicals against their concentration gradient
Exp proton pump
Secondary active transport
A carrier proton moves chemicals against their concentration gradient created by primary active transport
Exp: sucrose / H* conransporter
Sodium Potassium Pump
How does it work?
Bulk Transport
Using ATP, moves either large-size chemicals or large quantities of chemicals across the cell membrane using vesicles.
Exocytosis
Takes chemicals out
Exp: when Golgi apparatus produces secretory vesicles filled with proteins that are released out of the cell
Endocytosis
Brings chemicals in
Exp:
Phagocytosis
Brings in large-sized substances into the cell
Pinocytosis
Brings in large quantities of solutes into the cell
Receptor-mediated endocytosis
Brings in substances once a trigger (single molecule) active the reception on the cell membrane, which could be the solute itself
UNIT 6
Metabolism
All the reactions taking place in an organism
Anabolism
Anabolic rxn
Building up large molecules
Using dehydration rxn
Catabolism
Catabolic rxn
Breaking down large molecules
Using hydraulic rxn
The first law of thermodynamics:
Energy is neither created nor destroyed; instead, energy changes from one form to another
The second law of thermodynamics:
Systems are not efficient; energy is always lost to the surroundings, resulting in disorder, which is called entropy, measured in temp
Living things are constantly working against entropy by trying to maintain their homeostasis, a constant internal environment. We fight disorder and when we begin to lose, we start to age & die.
Energy is the ability to do work.
Forms of energy
Kinetic: increate in KE → inc heat
Potential: stored energy located in the bonds; stronger bonds = more energy
Heat: the amount of collisions particles form with each other & surroundings
Temperature: the average movement of particles
Different energy forms of chemicals
Ordered with high energy bonds; very reactivity
Exp: ATP dec Δs
Ordered with low energy bonds; low reactivity
Exp: glucose, glycogen, lipids dec Δs
Disordered with low energy bonds: low reactivity
Exp: ADP, CO2 Inc Δs
Energy Graph
The most common chemical used in biology is ATP
ATP → ADP + Pi
Adenosine triphosphate → adenosine diphosphate + inorganic phosphate
Coupling reactions
When two rxn are paired together, one is exergonic, supplying energy to the second rxn, which is endorgonic, allowing it to occur.
Cellular Work
Most common forms of cellular work
Chemical Work:
Definition: Chemical work involves the synthesis of complex molecules, the breakdown of larger molecules into simpler ones, and the conversion of one type of molecule into another.
Example: An essential example of chemical work is the synthesis of adenosine triphosphate (ATP), which serves as the primary energy currency of cells. During cellular respiration, cells break down glucose into carbon dioxide and water, releasing energy that is used to produce ATP.
Transport Work:
Definition: Transport work involves the movement of substances across cellular membranes. This can include the active transport of ions or molecules against their concentration gradient, requiring energy input.
Example: The sodium-potassium pump is a classic example of transport work. This pump actively transports sodium ions out of the cell and potassium ions into the cell against their respective concentration gradients, using energy derived from ATP hydrolysis.
Mechanical Work:
Definition: Mechanical work involves the physical movement or mechanical manipulation of cellular structures. This includes activities such as muscle contraction and the movement of cilia and flagella.
Example: Muscle cells perform mechanical work during contraction. The interaction between actin and myosin filaments, powered by ATP, leads to the shortening of muscle fibres and the generation of mechanical force.
Enzymes
Proteins (sometimes RNA) that lower the Ea to allow reactions to happen faster
How is the Ea lowered?
Enzyme brings reactants close to each other
Brings reactants together in the correct orientation
Places stress on bonds, allowing them to break and form more readily
Catalytic cycle of enzymes
Substrates (reactants) enter into the active site of the enzyme in the correct orientation
The active site is a specific region of the enzyme where the reaction takes place
receptors/transport proteins do now have an active site; they have a binding site instead. the binding site is for attachment, holding things
Each enzyme is specific to a substrate that is called the lock-and-key mechanism
Substrates are attached to the active site, and as soon as they enter it, the active site will wrap tightly around the substrates; this is called induced fit
Once substrates are in the enzyme, the enzyme-substrate complex is formed, where the substrate is held by weak intermolecular force in the active site
The enzyme lowers the Ea by placing stress on the bonds of the substrate using the weak intermolecular forces
The stress on the bonds created by the active site will allow bonds to break and new ones to form, generating products.
Products are repelled by the active site, causing them to leave the enzyme
Enzyme returns back to its original conformation, ready to receive a new substrate
Other chemicals can help enzymes function, such as…
Inorganic chemicals called cofactors
Exp: zinc, manganese, copper
Organic chemicals called coenzymes
Factors that disrupt enzyme function (all stress)
Pressure
Temperature
pH
Salt concentration
Electricity
poison/inhibitors
These change the structure of the enzyme, resulting in denaturation
Forms of enzymes
In the active form (more common in bio)
Always function and must be turned off when not needed (inhibited)
Inactive form
Produced in a nonfunctioning form and must be turned on or activated
Controlling the function of active enzymes
Inhibitors are chemicals that will inactivate enzymes
Two types…
1. Competitive inhibitors
Will compete with the substrate for the active site
This can be controlled by changing the substrate concentration
More substrate, lower chance of activation
Reversible competitive inhibitor
Inhibitor temporarily attaches to the active site and can leave
Irreversible competitive inhibitor
Permanently attaches to the active site and will not leave
2. Non-competitive inhibitors
Will attach to the enzyme at another location called the allosteric site, causing the active site to change in shape so that substrates will not fit properly
Or blocks the active site so substrates can’t enter
Exists as reversible and irreversible
Reactions are constantly regulated by feedback.
Positive
Feedback activation
As more products are created, this causes enzymes to work more and produce even more products
Negative
Feedback inhibition
As products increase in amount, the enzyme will be inactivated
The concentration of the product is the inhibitor
Example: threonine (amino acids) changes by a series of reactions to isoleucine, the isoleucine attaches have an allosteric site, inhibiting the cell. When isoleucine is used up, it will detach.
UNIT 7
Overview:
Energy flows in a linear function; compounds are going to cycle between photosynthesis and cellular respiration
An ecosystem is made out of living things as well as nonliving things, with the main light source being the sun.
Photosynthesis needs sunlight as a fuel and then releases organic molecules and O2 as waste. The waste then acts as fuel for cellular respiration with the release of ATP for cellular work. Which has a waste product of CO2 and H2O which gets used up by photosynthesis, creating a cycle.
Heat energy cannot be recycled.
Entropy is being generated in two ways, waste energy, and cellular respiration waste products.
Simplified chemical formula for cellular respiration
6O2 (g) + C6H12O6(aq) → 6CO2(g) + 6H20(l)
Coupling reactions:
ADP + Pi → ATP
Gets energy from Glucose, creating potential and chemical energy
Redox Reactions:
Split into 2 parts, oxidation & reduction
OIL: oxidation is lost; losing an electron
RIG: reduction is gained; gain of electron
Performed by dehydrogenase, a coenzyme that helps the reaction occur
In the case of the formula, oxidation is happening from oxygen to carbondioxide and reduction is happening from glucose to water
Phosphorylation
When a chemical gains a phosphate group
Two types:
Substrate level phosphorylation
When a phosphate group form an organic molecule is picked up by another organic molecule
Oxidative phosphorylation
When a chemical gains a phosphate group using the energy from the oxidation of another chemical
Glycolysis
Overall reaction
Takes place in the cytoplasm
Glucose →→→→→→ 2 pyruvates
Creates:
2 ATP by using 2 ADP & Pi
2 NADH & H* by using 2 NAD*
Ten RXNS of glycolysis
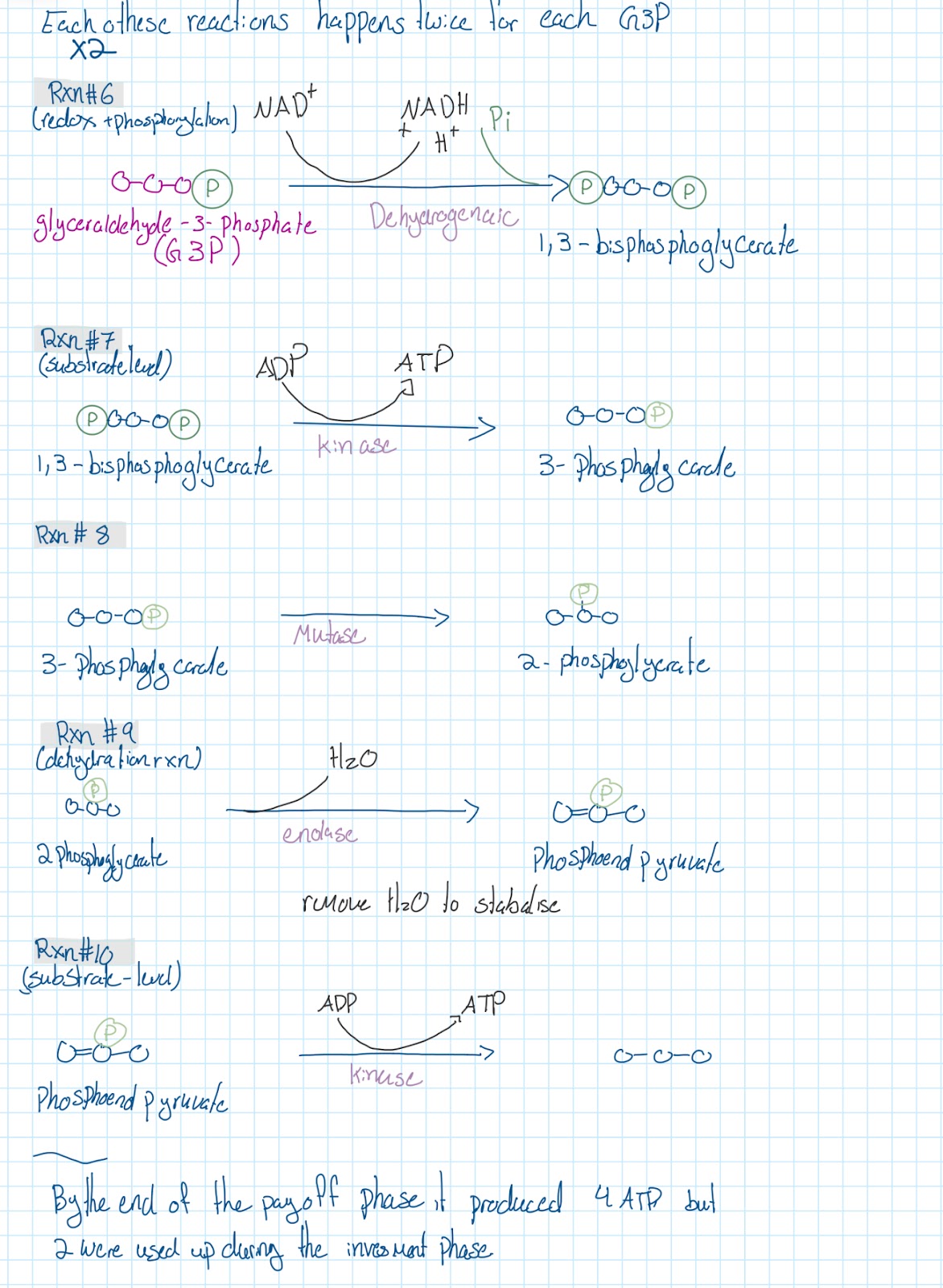 Linkage Reaction
Linkage Reaction 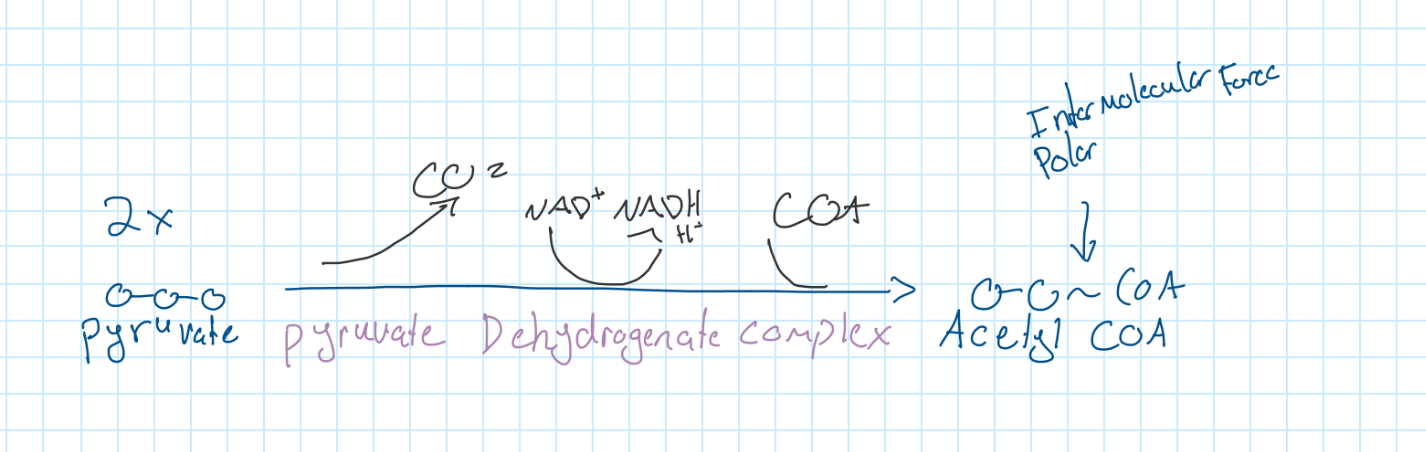
Krebs Cycle
The goal of the krebs cycle is to trap as much energy as possible form Acetyl CoA in NADH. FADH2 & ATP
Two e- carrieres
NAD* + 2e- + 2H* → NADH + H*
Nicotinamide Adenine Dinucleotide
FAD + 2e- + 2H* → FADH2
Flavin Adenine Dinucleotide
For one glucose molecule
Stage | ATP | NADH | FADH2 | CO2 |
Glycolysis | 2 | 2 | 0 | 0 |
Link | 0 | 2 | 0 | 2 |
Krebs | 2 | 6 | 2 | 4 |
Total | 4 | 10 | 2 | 6 |
Electron Transport Chain
Poisons
Rotenone:
Attaches to complex 1, stopping the flow of electrons, kills you
Cyanide:
Attaches to complex 4, preventing the flow of electrons, stops the reduction of O2. it is irreversible, and can kill anyone with small amounts
Carbon monoxide:
Attaches to complex 4, preventing the flow of electrons, stops the reduction of O2. reversible if caught early enough
DNP:
Creates holes in the phospholipid bilayer, disrupting the H+ gradient. In large amounts can kill the person, overall stop the flow of ATP
Oligomycin:
Stops ATP synthase, stopping the flow of H+, which stops the creation of ATP
Producing Energy in the absence of O2
ADP & Pi → ATP
Glucose →→→→→→ 2 pyruvates
NAD* → NADH + H*
If there is sufficiant oxygen, it goes through cellular/Aerobic respiration in the mitochondria
If not, it goes through fermentation, anaerobic respiration
Fermentation
Lactic Acid
Done by humans/animals/bacteria/fungi
Lactic acid is a warning mechanism informing you that you are out of oxygen
Alcohol
Done by plants/bacteria/fungi (yeast)
UNIT 10
Cell division When a cell divides, or splits in half, in order to create new cells
Reproduction when an organism creates new off springs
Organism
Unicellular:
when they reproduce, they perform cell division. The mother cell divides into two daughter cells
Multicellular:
Asexual; involves one parent, produces clone with no genetic difference
Sexual: involves two parents, produces offspring with egentic differences
Performs cell division for
Repair
Growth
Maintenance
Budding: when a unicellular organism has a growth appearing in one side that develops into a new individual and falls off; exp: Hydra
Yeast
When conditions are favourable, yeast will reproduce by budding
When conditions are not favourable, yeast will produce sexually
Why? If they aren’t doing well and the condition is bad, they don’t want to clone themselves because they know their offspring will have the same bad condition. If they reproduce sexually, there becomes a chance that the mix of genetics can create a version that will survive the condition
Fragmentation: when a piece of an animal is cut off, and it will grow to become a new individual, exp, star fish
Vegetative propagation: a survival mechanism where a broken piece of plant will grow to become its own individual
Chromosomes
A molecule of DNA (double helix)
Prokaryotic,
Each prok cell will have one circular DNA molecule
Eukaryotic,
Each euk will have several linear DNA double helices, each one wrapped around groups of proteins → histones to create chromosomes
When one chromosome is duplicated, it becomes a set of sister chromatids held together at the centromere, the centre point.
When it is duplicated, the information is no longer accessible
Forms of DNA depending of cell activity
When a cell is NOT dividing
DNA exists in the form of chromatin, the chromosomes are spread out, and the information is easily accessible
When a cell IS dividing
DNA exists in the form of chromosomes that are condolences, wrapped up tightly. Information is no longer accessible
Cell cycle
The most basic function of the cell cycle is to duplicate accurately the vast amount of DNA in the chromosomes and then segregate the copies precisely into two genetically identical daughter cells.
Steps of the cell cycle
Interphase
G1 phase, first gap
S Phase, synthase phase
G2 phase, second gap
Celldivision→ mitotic phase
Prophase
Prometa phase
Metaphase
Anaphase
Telophase
Cytokinesis
Functions in each step:
First Gap
Brings in lots of nutrients
Generates lots of energy
Organelles/proteins & other structures will duplicate
Cells grow in size
Synthase phase
DNA is duplicated
Second Gap
Everything in the first gap will continue if not finished
Checks and corrects any error found in DNA
If the error cannot be fixed, the cell enters apoptosis; which is cell suicide
Pro phase
DNA condenses from chromatin into duplicated chromosomes
The nuclear envelope disintegrates, allowing access to the information
Centrioles begin to move away from each other to opposite poles
Spindle fibres, microtubules, begin to form. (from the cytoskeleton)
Prometa Phase
Nucleus disappears
Centrioles continue to move to the opposite pole
Kinetachrome fibres from each centrosome connect to the centrosome of each duplicated chromosome and begin to move them to the equator of the cell
Meta Phase
Duplicated chromosomes are lined up at the equatorial plane
Centrioles have reached opposite poles
Ana Phase
Kinetochore fibres shorten, pulling sister chromatids apart & moving the chromosomes away from each other
Non-kinetochore fibres lengthen, stretching the cell
Tela Phase
The appearance of the cleave furrow indicates the start of cytokinesis
Spindle fibres disintegrate
Formation of nuclear envelope around each set of chromosomes
DNA unwinds from chromosomes and into chromatin
Mitosis
Duplication of the nucleus
Cytokinesis
The division of the cytoplasm of the cell
Animal cells
rely on a belt of proteins known as the contractile ring, which is made from actin
Plant cells
does not rely on a contractile ring because the cell is way too rigid.
Golgi apparati at each end of the cell will produce transport vesicles that fill with pectins that move to the centre of the cell
at the centre of the cell, the transport vesicles fuse with each other to create the cell plate
the cell plate lines up in the middle of the cell to become the new cell wall
the cell plate continues to grow and reach the cell membrane to become the cell membranes of the two new cells, the cells will be attached by the middle lamella that is filled with pectin
cellulose is secreted by each new cell to create its own cell wall
Drawing of the cells;
Factors that are controlling the cell cycle
space is required around cell for them to divide; density-dependence
in tissues, cells form layers and not clumps; cells do not grow on top of each other
a rigid surface is needed for attachment before cells can divide; anchorage dependence
signal molecules called growth factors inform cells that they need to enter the cell cycle