🧬 AP Biology Unit 1
8-11% AP Weighting
1.1 Structure of Water and Hydrogen Bonding
1.2 Elements of Life
1.3 Introduction to Biological Macromolecules
1.4 Properties of Biological Macromolecules
1.5 Structures and Functions of Biological Macromolecules
1.6 Nucleic Acids
Basics of Life
Click Here for a video on the Characteristics of Life
Click Here for a video on Taxonomy (kingdoms/domains of life)
Emergent Properties are characteristics of organisms that are the result of several systems interacting in an organism. It shows that in biology the whole is greater than the sum of the parts.
The Characteristics of Life (specific phrasing changes from source to source)
Reproduce
Grow and Change
Respond to Stimuli
Adapt
Have an ordered structure, be composed of cells
Process energy
Maintain Homeostasis
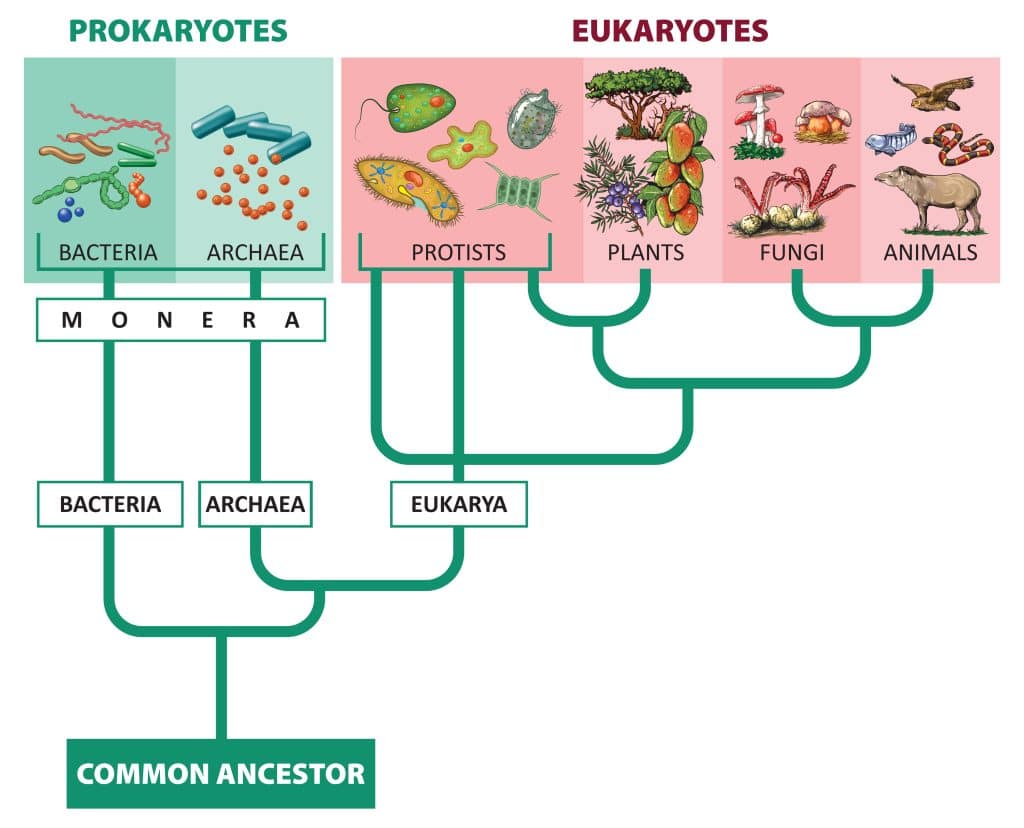
Structure and function are related. If structure of something, like a protein, is wrong then it won't be able to complete its function. There are 3 taxonomic domains of life, Bacteria, Archaea, and Eukarya. Eukarya are the only eukaryotes (cells that include a nucleus and membrane-bound organelles). The kingdoms within Eukarya are Protists, Plants, Fungi, and Animals.
1.1 - Water
Click Here for a video on the Properties of Water
Properties of Water
Adhesion
Cohesion
Universal Solvent
Capillary Action
Water is less dense as a solid
Surface Tension
High resistance to temperature changes (High Specific Heat)
Adhesion is when water sticks to other molecules. Cohesion is when water sticks to other water molecules. Water is the Universal Solvent Because it dissolves molecules with covalent bonds. Capillary Action is when water “climbs” up a tube. (Like xylem) Water is less dense as a solid. (Ice floats in water) Surface Tension is the idea that water molecules will try to stick together to form the smallest possible surface areas. High resistance to temperature changes (High Specific Heat) explains that it can take more heat to warm water than other things like metal.
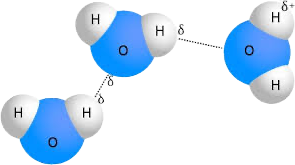
Water is polar, meaning it has an unequal distribution of charge. This causes hydrogen bonds to form, and hydrogen bonds cause the properties of water. Hydrogen bonds are where the slightly negative oxygen is attracted to the slightly positive hydrogens of another water molecule. These are not real bonds. Nitrogen atoms bonded to hydrogen can also have hydrogen bonds. (NHx)
1.2 - Chemistry/Elements of Life
There are four major elements in the body:
Hydrogen
Oxygen
Nitrogen
Carbon
Valence is the number of electrons an ion can share. Hydrogen has a valence of 1, Oxygen has a valence of 2, Nitrogen has a Valence of 3, and Carbon has a valence of 4. Valences shells further from the center of an atom have higher energy.
For atoms, opposites attract, resulting in ionic bonds between a positive and negative ion. An electron is transferred in ionic bonds. They are strong but dissociate in water, so they are rarely used in biology. This is why water is called the Universal Solvent. Covalent bonds share an electron. (Or multiple)
Organic Chemistry is the study of carbon compounds. Carbon is the backbone of life because it has a valence of 4. Organic compounds can be simple, like CO2, or much more complex.
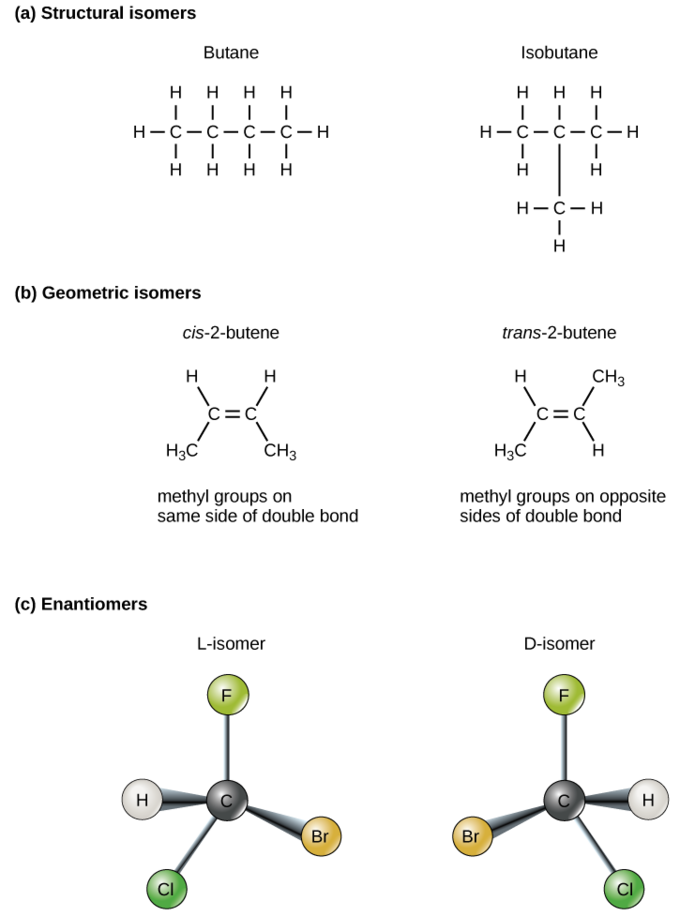
Isomers are compounds that have the same formula (same number and type of molecules) but have different structures and therefore have different chemical properties. Structural isomers are molecules with the same formula but different covalent arrangement of atoms. Geometric or cis-trans isomers are compounds with the same structure but the atoms in the structure can be in different spaces. Enantiomers are molecules that are mirror images, and generally one is inactive.
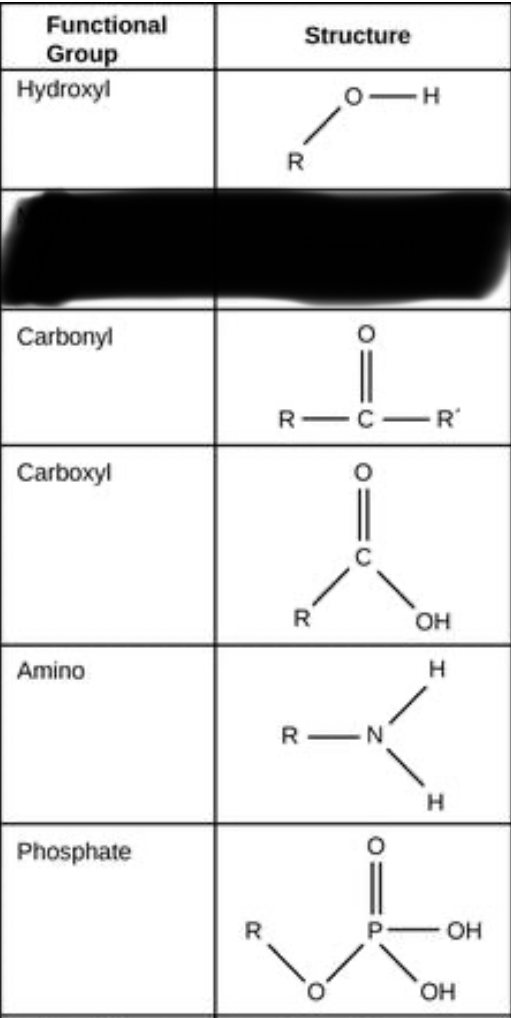
There are several different functional groups:
Hydroxyl (OH): These compounds are generally alcohols are end in -ol
Carbonyl (CO): An oxygen double bonded to a carbon skeleton/chain.
Carboxyl (COOH): A carbon chain with a single bonded O and OH. (If you put an Ox in a box it goes COOH. It’s stupid but it works if you had your teacher scream COOH in the middle of class.)
Amino (NH2): A nitrogen bonded to two hydrogen atoms and a carbon chain.
Phosphate (OPO3): Generally any phosphate and oxygen bonded will be a phosphate group.
1.3-1.5 - Macromolecules
Click Here for a video on Macromolecules
There are four biomolecule classes, protein, lipids, carbohydrates, and nucleic acid. All but lipids form chain-like molecules called polymers which are made of monomers. Monomers are combined via dehydration synthesis, in which a water molecule is removed. Monomers must be broken apart with hydrolysis, which requires water as a reactant. It is important to know what lyse means to break down or break apart and this can also help remember the function of hydrolysis.
Structures of Macromolecules:
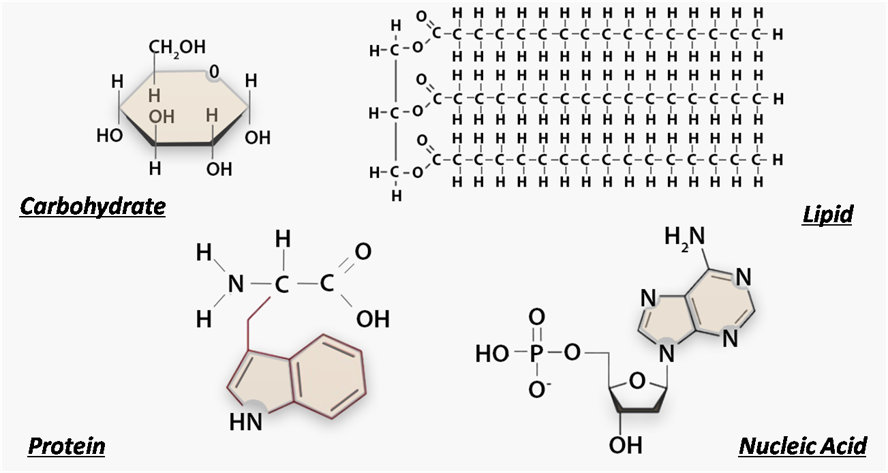
Carbohydrates
Monomer - Monosaccharide:
Polymer - Polysaccharide
Bond - Glycosidic linkage, covalent
Purpose - Fuel and building materials
Made of - C, H, O
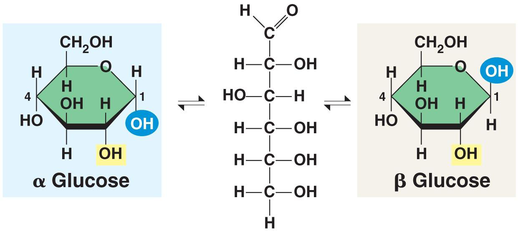
The suffix’s -ose, glyco-, and -saccharide all indicate a molecule is a sugar. The most common monosaccharides is glucose. Most monosaccharide have a molecule formula that is a multiple of CH2O. Sugars are shown as hexagons. Starch and Glucose is “squiggly,” but the sugars in plant cell walls are straight. This is due to the arrangement of the molecules, either beta or alpha glucose. Alpha glucose is for animals and plants, and beta glucose is used in plants and cannot be broken down by the digestive system.
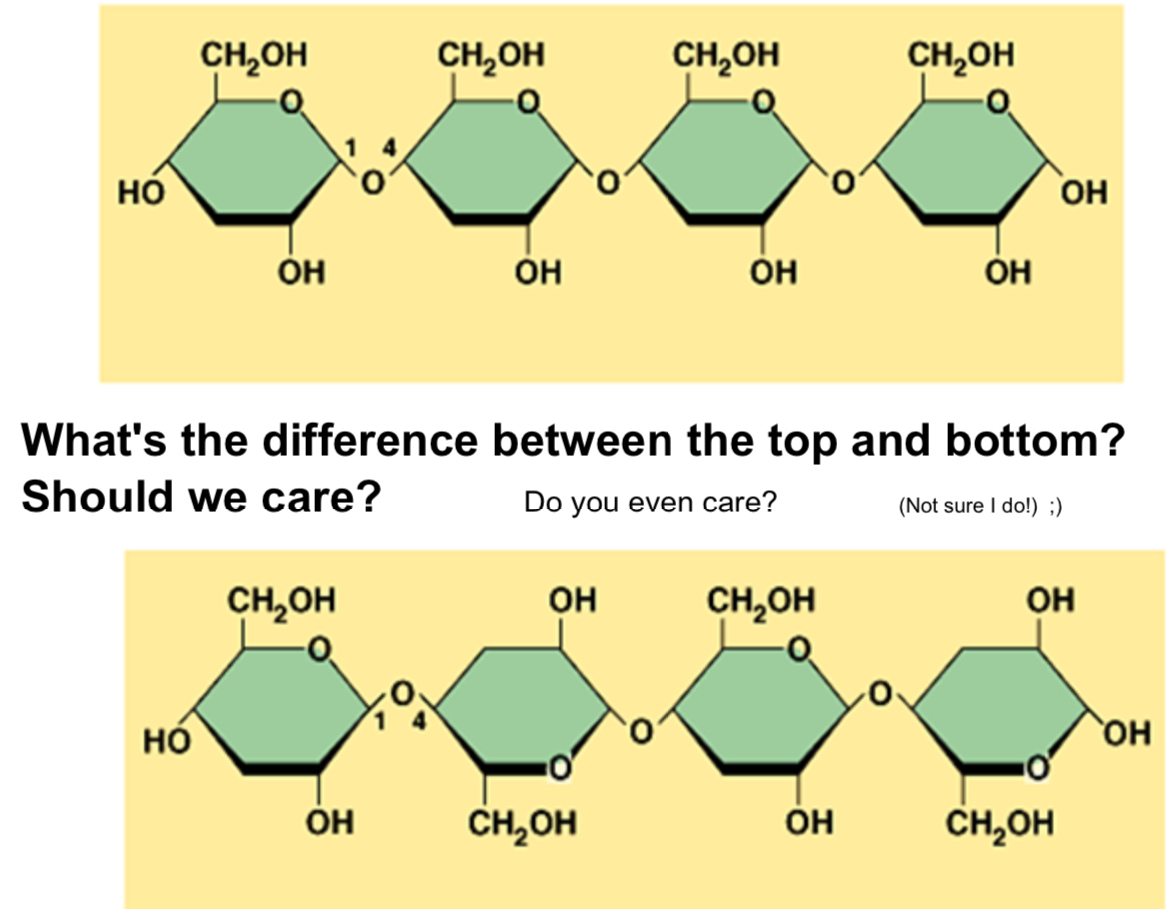 (Clearly my teacher has a lot of love for this unit)
(Clearly my teacher has a lot of love for this unit)
Glycogen: Used in animals and is broken down to release glucose when sugar is needed. It is a type of alpha glucose and is used for storage.
Starch: Used as storage in plants and is made of alpha glucose.
Cellulose: Made of beta glucose and is used as a building material
Chitin: Used in the cell walls of fungi and the exoskeleton of bugs. It is structural.
Lipids
Monomer: Glycerol
“Polymer”: Polyglyceride, triglyceride
Bond: Ester Linkage
Purpose: Insulation, storage, cushioning, communication (hormones), and plasma membranes.
Made of: C, H, O
Lipids are not made of true polymers as each polyglyceride is made of 3 fatty acid chains held together by a glycerol, which is different from other polymers. Saturated fatty acids are straight, and unsaturated fatty acids are bent. Lipids are hydrophobic.
There are three types of lipids
Fats - store energy, insulate, and cushioning
Phospholipid - Component of the cell membrane
Steroids - Cholesterol and hormones such as estrogen
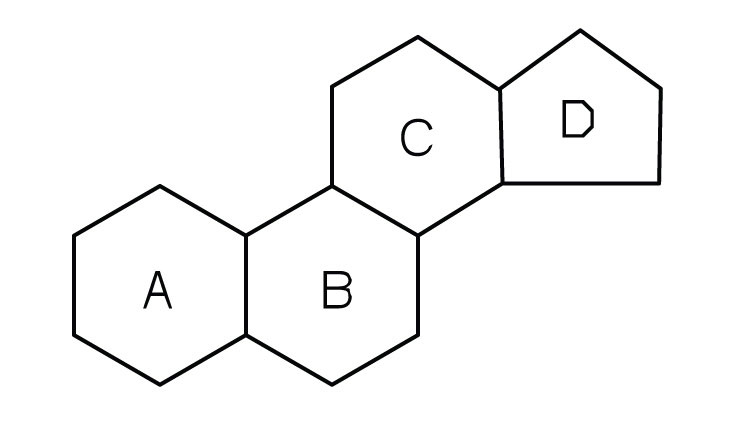
Steroids are shaped like three honeycombs and a house. Hormones are made of cholesterol.
Proteins
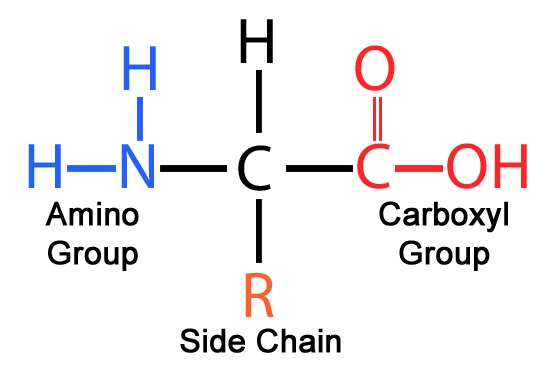
Monomer: Amino Acids
Polymer: Polypeptide
Bond: Peptide bond
Purpose: Basically everything
Made of: C, H, O, P
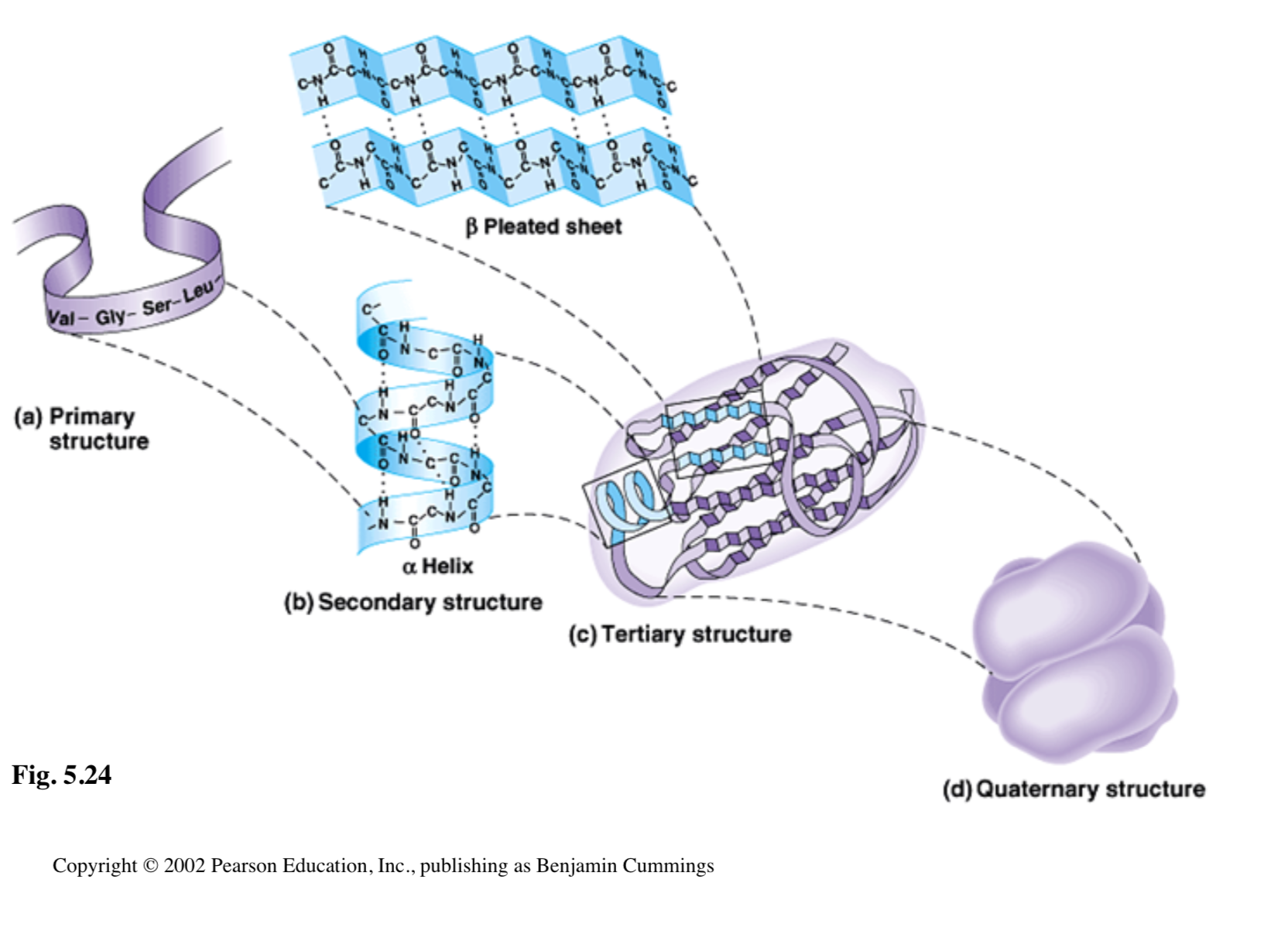
The shapes of proteins are integral to their function. If you have a misshapen key, it wont unlocked a lock. Protein Confirmation is another work for their shape. There are four confirmation structures:
Primary - The sequence of amino acids
Secondary - Hydrogen bonds causing beta pleated sheets or alpha helices
Tertiary - Interactions between the R (changeable) group, hydrophobic groups, vanderwall interactions, ionic bonds, and disulfide bridges
Quadanery - Not in all proteins, it is when multiple proteins combine to form a bigger protein.
Proteins denature in changes as the hydrogen bonds, ionic bonds, and disulfide bridges, but NOT to peptide bonds.
1.6 - Nucleic Acids
Why is this a separate section, as nucleic acids are a type of macromolecule? No clue! Ask college board.
Monomer: Nucleotide
Polymer: Nucleic acid
Bond: Phosphodiester bonds, hydrogen bonds
Purpose: Genetic information storage
Made of: C, H, O, N, P
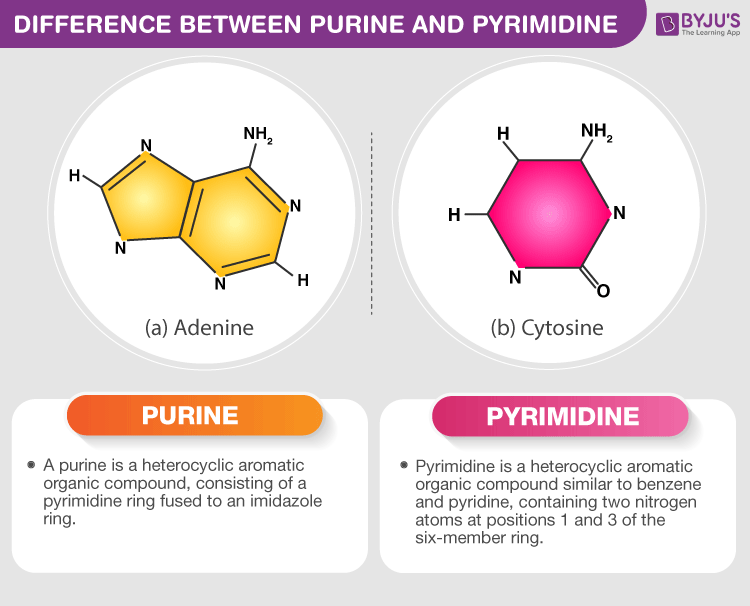
Nucleic acids make up DNA and RNA and store genetic information. One type, Adenine, is a part of ATP (energy). Another energy form is GTP, with Guanine, another nucleic acid.
Pyrimidine nucleotides: C, T, and U.
Purine nucleotides: A and G.