Chapter 3: Week 3: Carbohydrates
✩Carbohydrates✩
*Framework of biological molecules consists primarily of carbon bonded to
-Carbon
-O, N, S, P or H
*Can form up to 4 covalent bonds
*Hydrocarbons – molecule consisting only of carbon and hydrogen
-Nonpolar (no separation of charge, so no positive or negative poles are formed, H-F, 0=0)
-Functional groups add chemical properties
✧Macromolecules✧
TABLE 3.1 Macromolecules
| Macromolecule | Subunit | Function | Example |
|---|---|---|---|
| CARBOHYDRATES | |||
| Starch, glycogen | Glucose | Energy storage | Potatoes |
| Cellulose | Glucose | Structural support in plant cell walls | Paper; strings of celery |
| Chitin | Modified glucose | Structural support | Crab shells |
| PROTEINS | |||
| Functional | Amino acids | Catalysis; transport | Hemoglobin |
| Structural | Amino acids | Support | Hair; silk |
| NUCLEIC ACIDS | |||
| DNA | Nucleotides | Encodes genes | Chromosomes |
| RNA | Nucleotides | Needed for gene expression | Messenger RNA |
| LIPIDS | |||
| Fats | Glycerol and three fatty acids | Energy storage | Butter; corn oil; soap |
| Phospholipids | Glycerol, two fatty acids, phosphate, and polar R groups | Cell membranes | Phosphatidylcholine |
| Prostaglandins | Five-carbon rings with two nonpolar tails | Chemical messengers | Prostaglandin E (PGE) |
| Steroids | Four fused carbon rings | Membranes; hormones | Cholesterol; estrogen |
| Terpenes | Long carbon Chains | Pigments; structural support | Carotene; rubber |
✩Carbohydrates✩
*Carbohydrates are the most common type of organic compound
*A carbohydrate is an organic compound such as sugar or starch
*Used to store energy
*Like most organic compounds, carbohydrates are built of small, repeating units that form bonds with each other to make a larger molecule
*Carbohydrates contain only carbon, hydrogen, and oxygen
✩Organic Molecules✩
*Organic molecules contain carbon
*It can make up to 4 bonds
-Usually single or double bonds
*Carbon can form nonpolar and polar bond
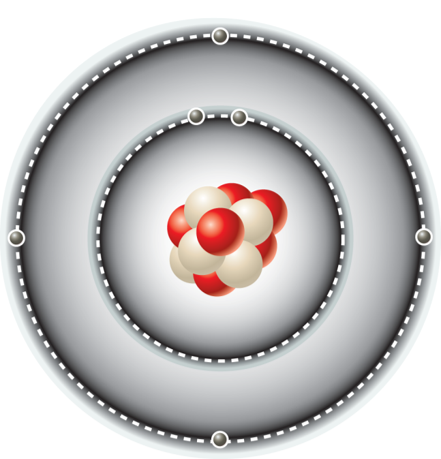
✩Macromolecules✩
*Condensation or dehydration reaction
-monomers---> polymers
*Hydrolysis
-Polymers--->monomers
*Four major types of organic molecules and macromolecules
1.Carbohydrates
2.Lipids
3.Proteins
4.Nucleic acids


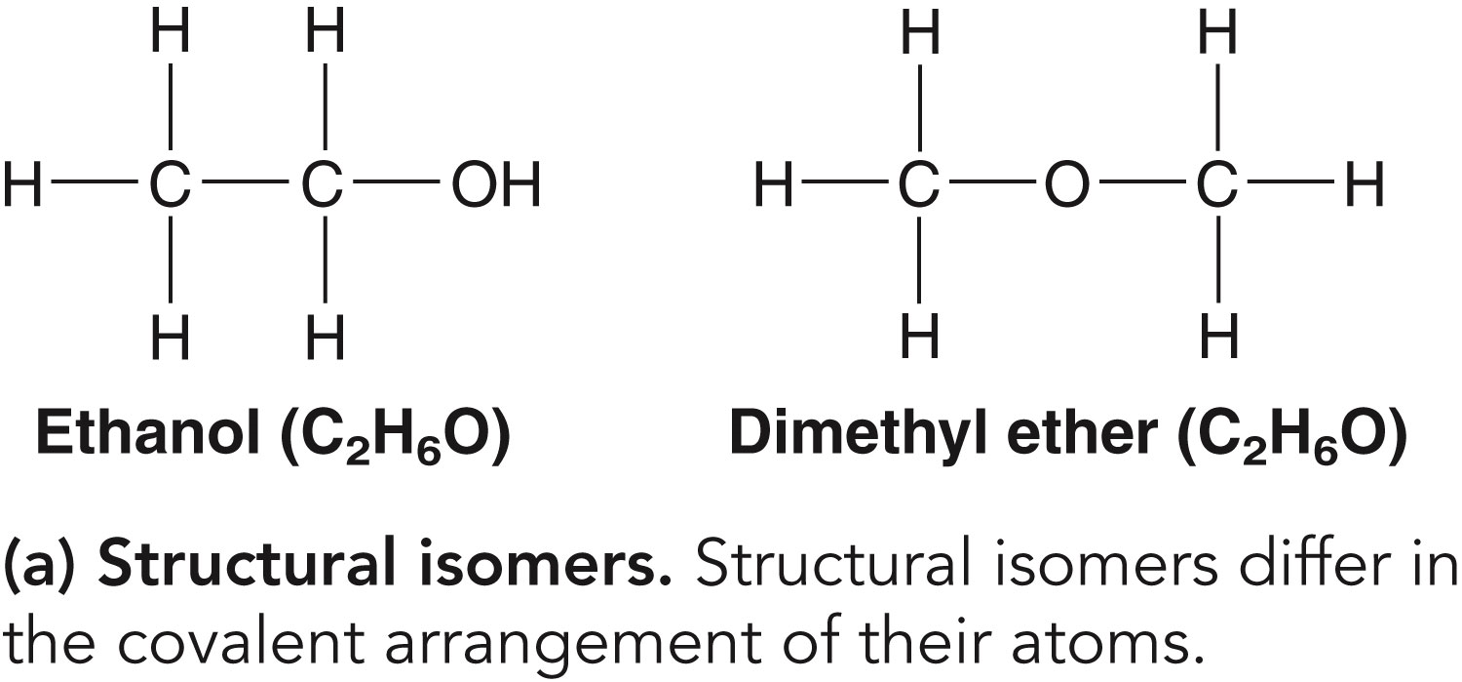
✩Isomers✩
*Compounds with the same molecular formulas but different structures and properties
*Usually, one isomer is biologically active, another is not
*Three types: structural isomers, geometric isomers, and enantiomers
*The same components can link in more than one pattern, generating a wide variety of molecular shapes
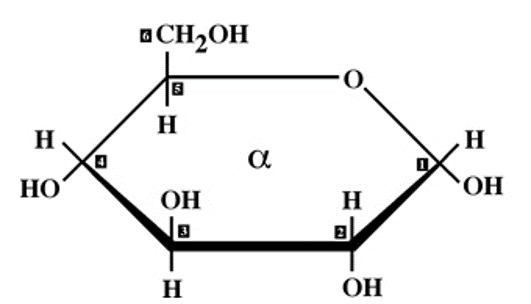
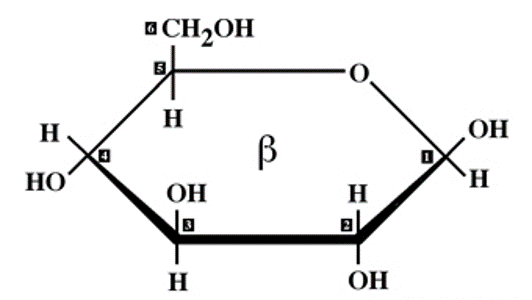
✩Structural Isomers✩
✩Geometric Isomers✩
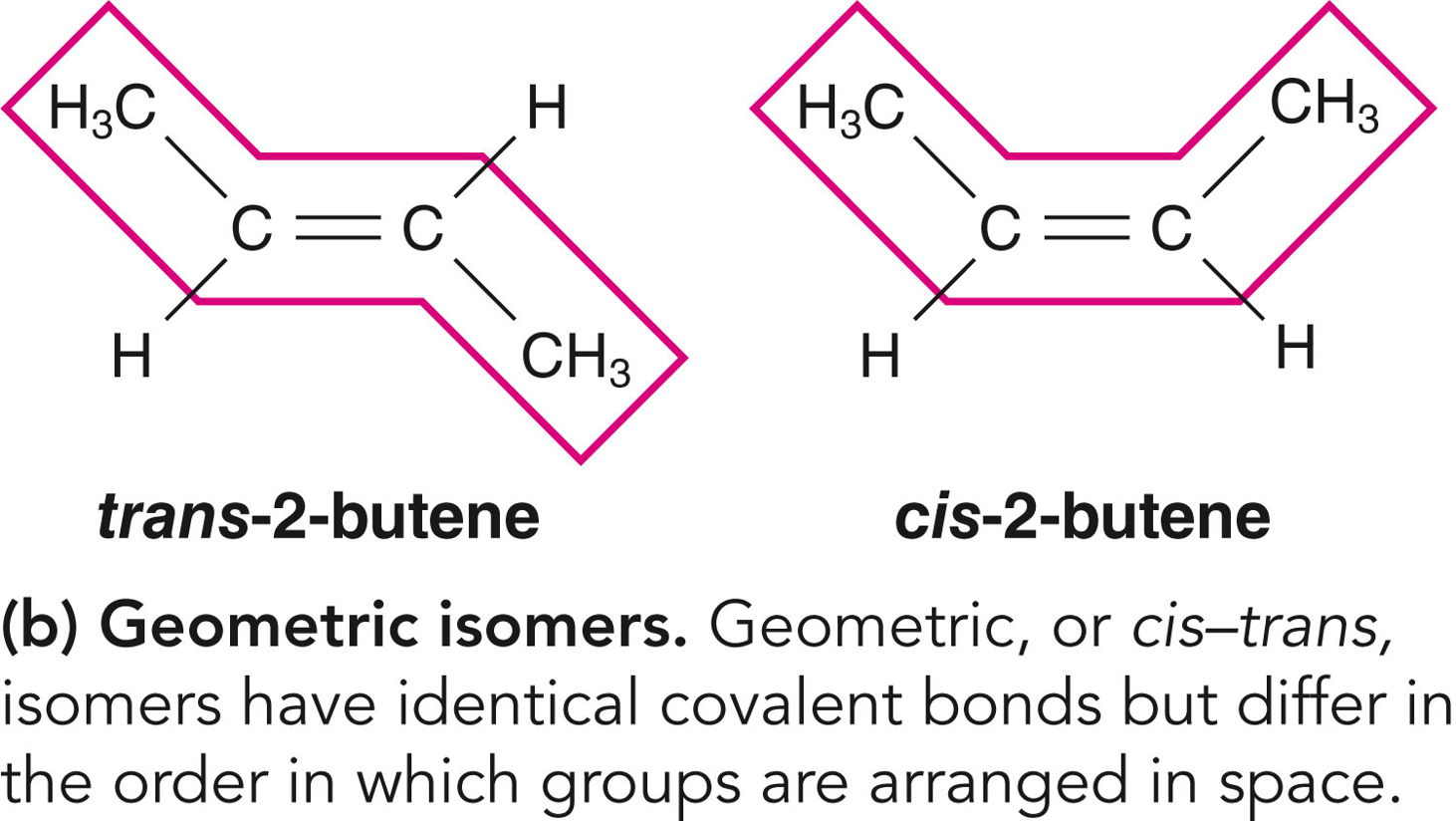
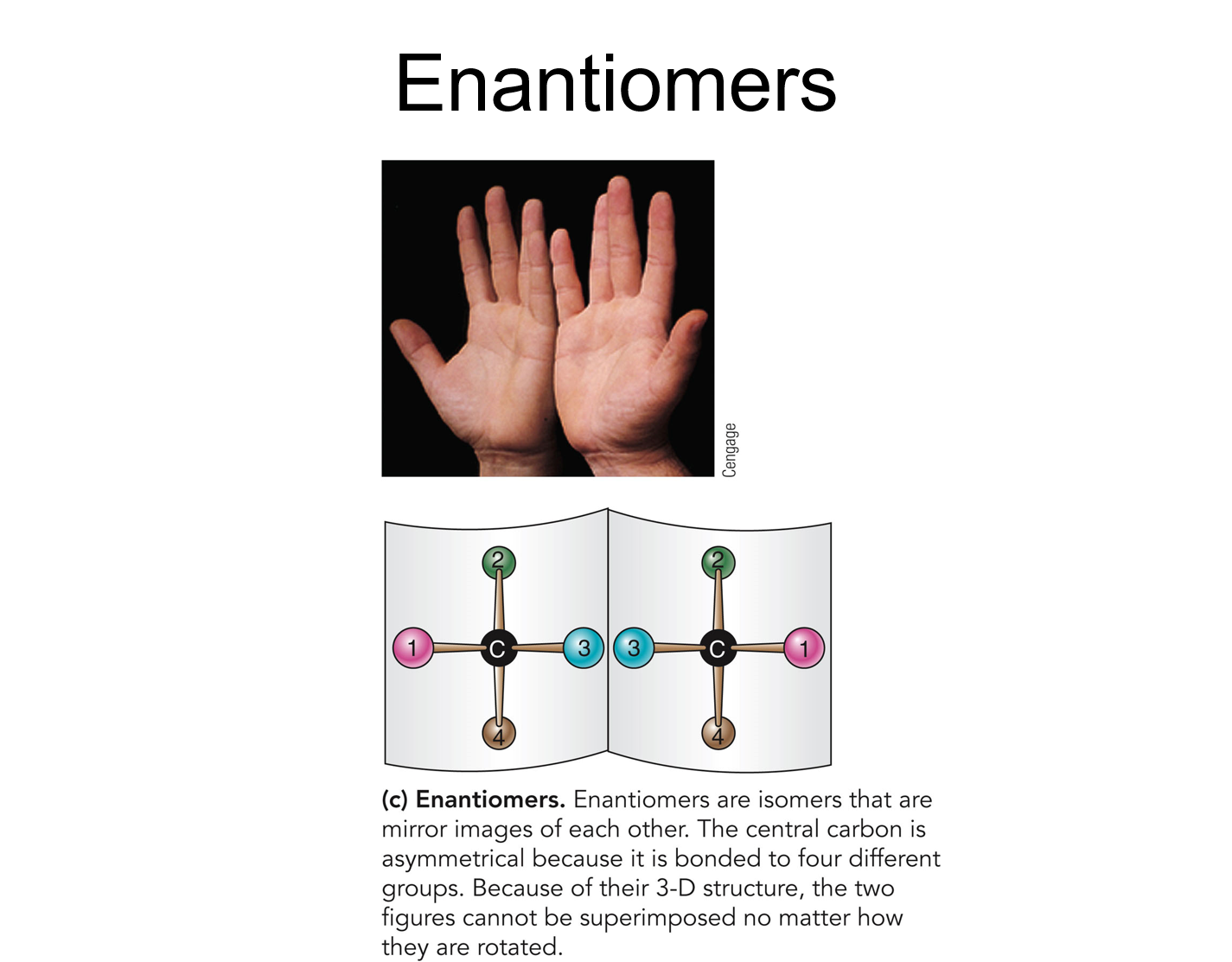
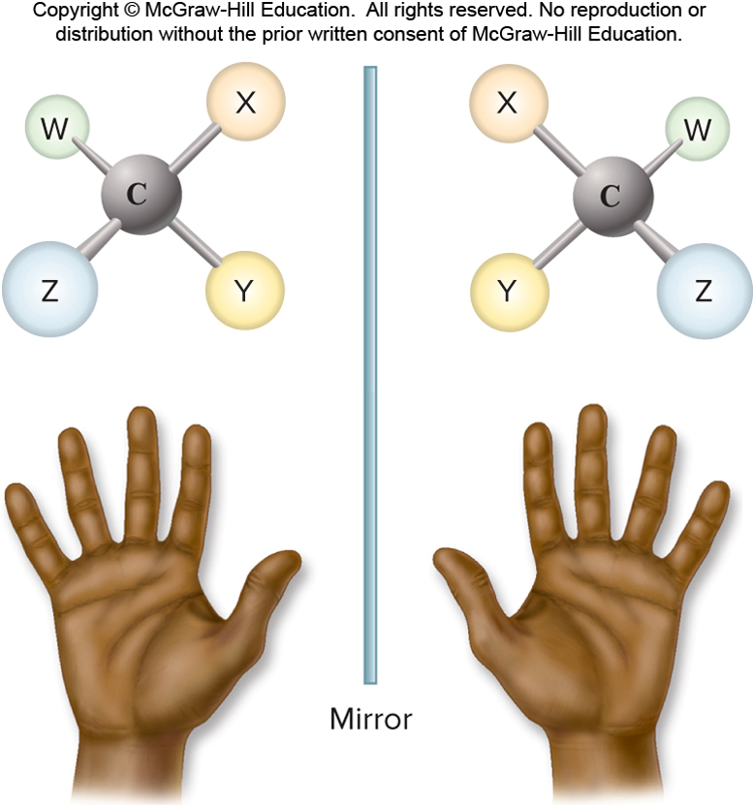
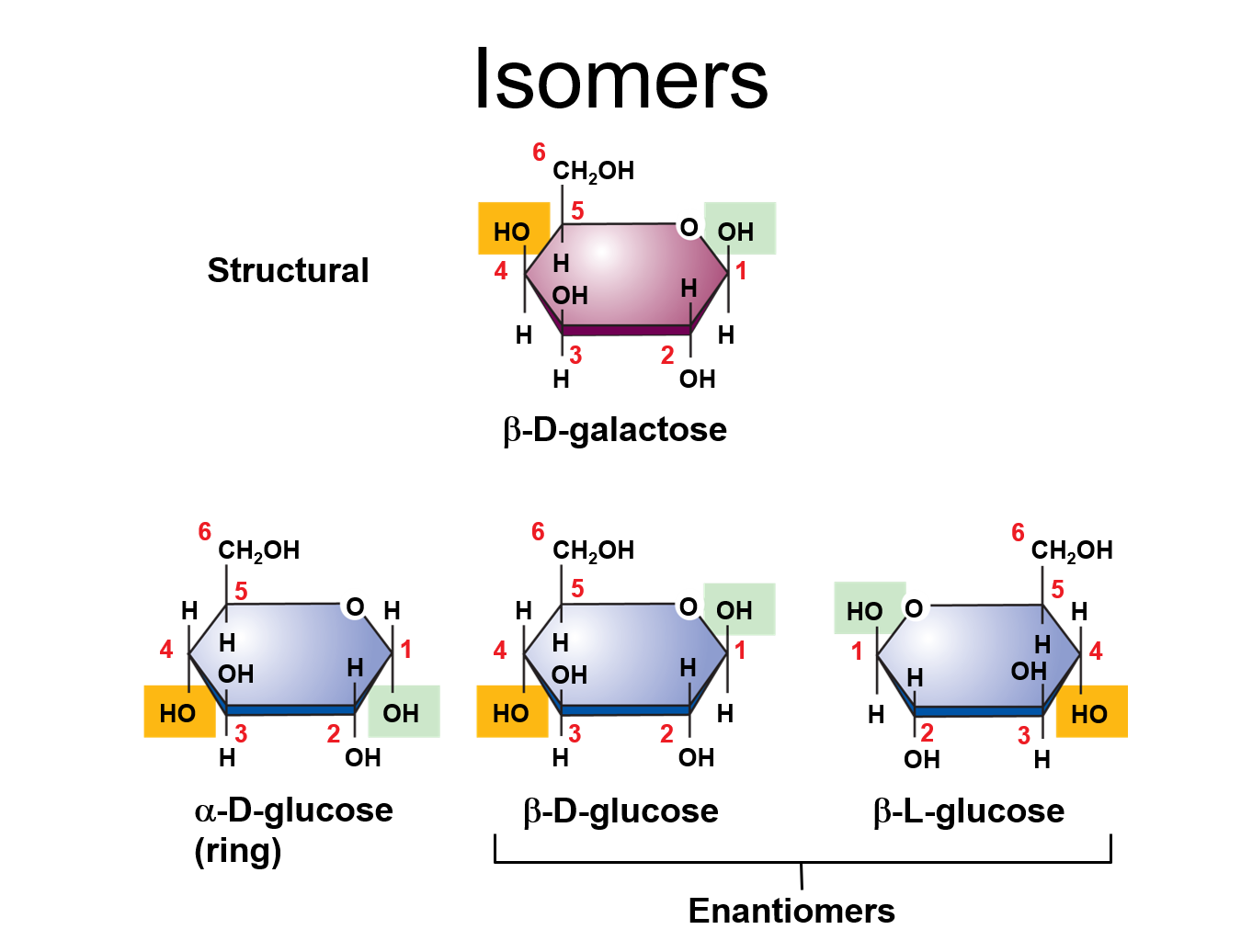
 ✩Carbohydrates✩
✩Carbohydrates✩
*Composed of carbon, hydrogen, and oxygen atoms
*Cn(H2O)n
*Monosaccharide---> Disaccharide---> Polysaccharide
✩Monosaccharides✩
*Simplest sugars
*Most common are 5 or 6 carbons
-Pentoses- ribose (C5H10O5), deoxyribose (C5H10O4)
-Hexose- glucose (C6H12O6)
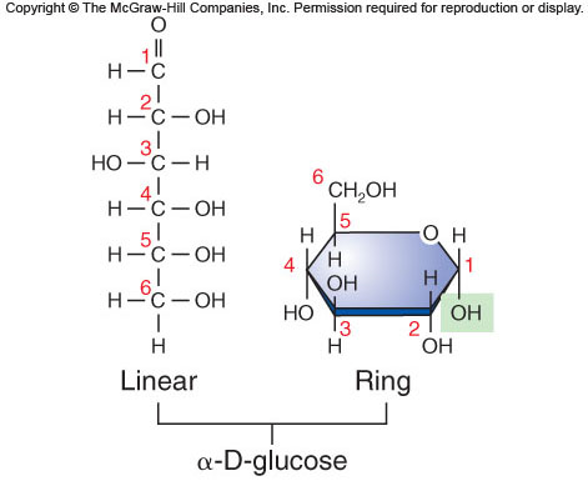
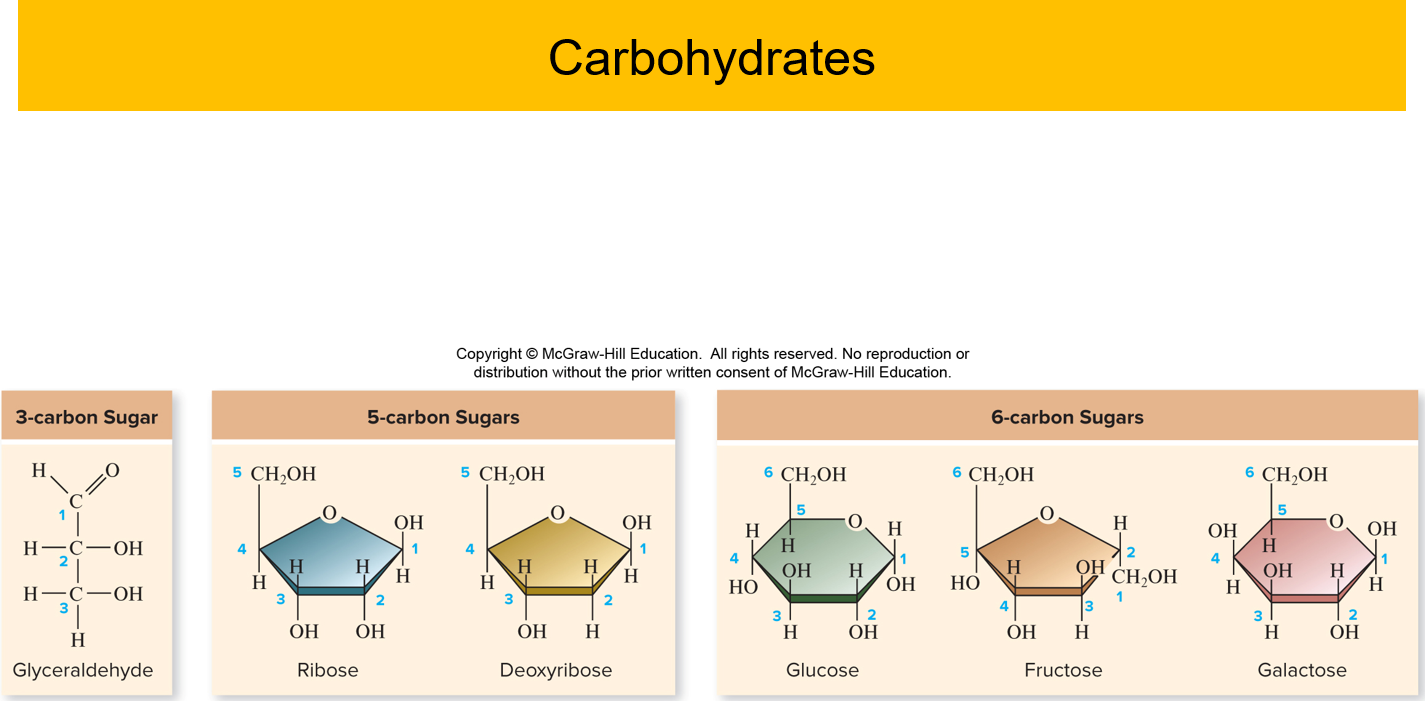
✩Monosaccharides✩
*Simplest carbohydrate
*6 carbon sugars play important roles
*Glucose C6H12O6
*Fructose is a structural isomer of glucose
*Galactose is a stereoisomer of glucose
*Enzymes that act on different sugars can distinguish structural and stereoisomers of this basic six-carbon skeleton
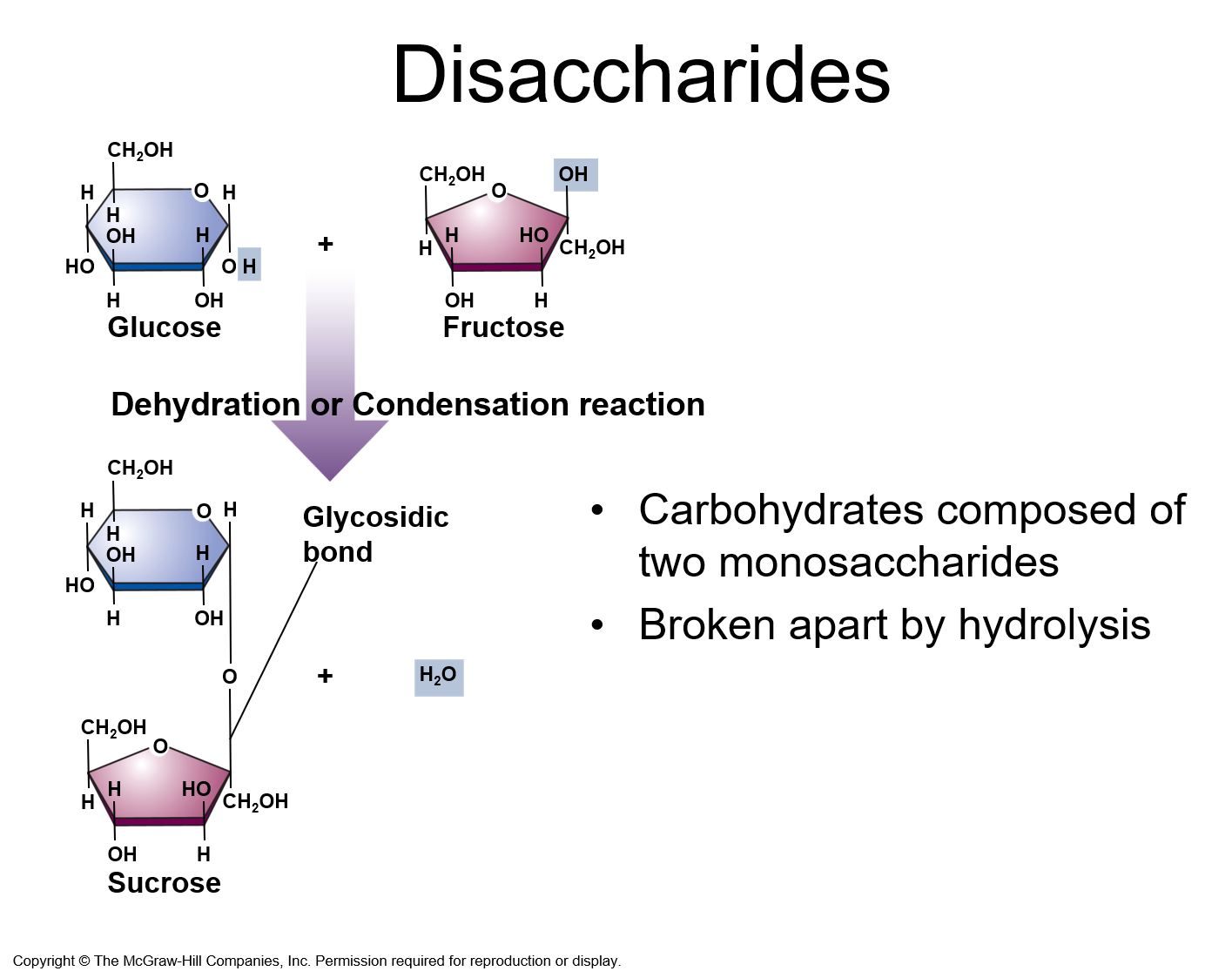
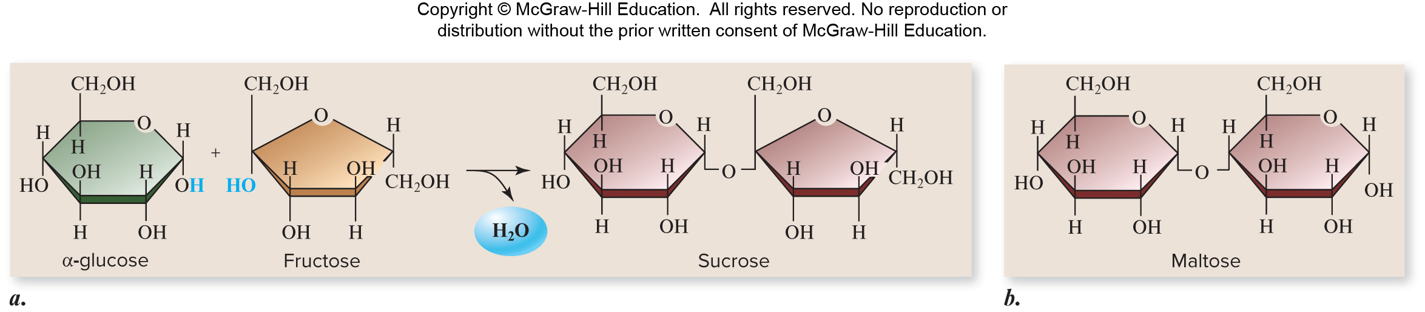
✩Disaccharides✩
*Used for sugar transport or energy storage
*Examples: sucrose, lactose, maltose
✩Polysaccharides✩
•Long chains of monosaccharides
•Many monosaccharides linked together to form long polymers
•Energy storage
–Plants use starch
–Animals use glycogen
•Structural support
–Plants use cellulose
–Arthropods and fungi use chitin
\n ✩Hydrolysis✩
•This is a reaction where a chemical bond is broken using a water molecule. During this reaction, a water molecule splits up into a proton and a hydroxide ion.
•In Chemistry, Hydrolysis is a chemical reaction with water, in which a macromolecule is separated into smaller molecules.
•Hydrolysis involves water to split polymers into monomers.
•Hydrolysis occurs when water is added to the equation to break it down or separate it.
•In our bodies, Hydrolysis Is the main process to release energy. When we eat food, it is digested or broken down into substances so the body can absorb it and convert it to energy.
•Foods, having complex molecules are broken down into simple molecules. When energy is needed for biosynthesis, ATP is hydrolyzed and stored energy is released for utilization.
\n \n \n 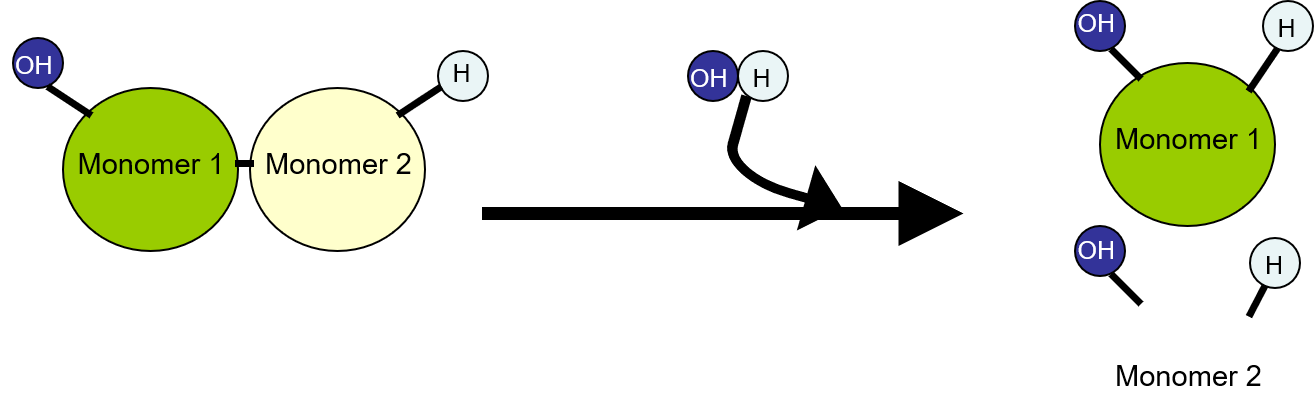 ✩Dehydration Synthesis✩
✩Dehydration Synthesis✩
•Dehydration Synthesis
“dehydration” = to remove water
“synthesis” = to make
The process of CREATING carbs, lipids, and proteins by removing water
•Dehydration means to take away water, and synthesis means to build or create something.
•Dehydration Synthesis is defined as taking away water to build something.
This process happens by removing one molecule of –OH (hydroxyl group) and one molecule of -H to form H2O or water. This results in covalently joining two monomers (small molecules) to form a polymer (larger molecule).
\n 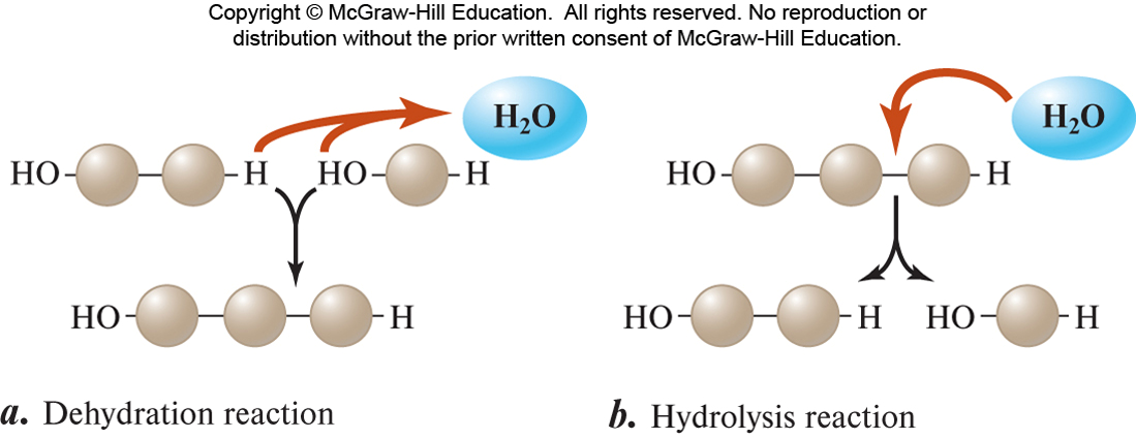
\n ✩Condensation Reactions✩
•A condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule(s) in the process.
•The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules.
•A condensation reaction is the formation of a larger molecule from two smaller ones, which also form another, smaller molecule by losing functional groups in order to join together.
•Condensation reactions are used to make crucial large molecules called macromolecules in the body, such as carbohydrates, lipids, and proteins. Carbohydrates are simple sugars such as glucose, which is used for energy in the body
•Result in loss of smaller molecule
•Dehydration: smaller molecule is water (H2O)
\n 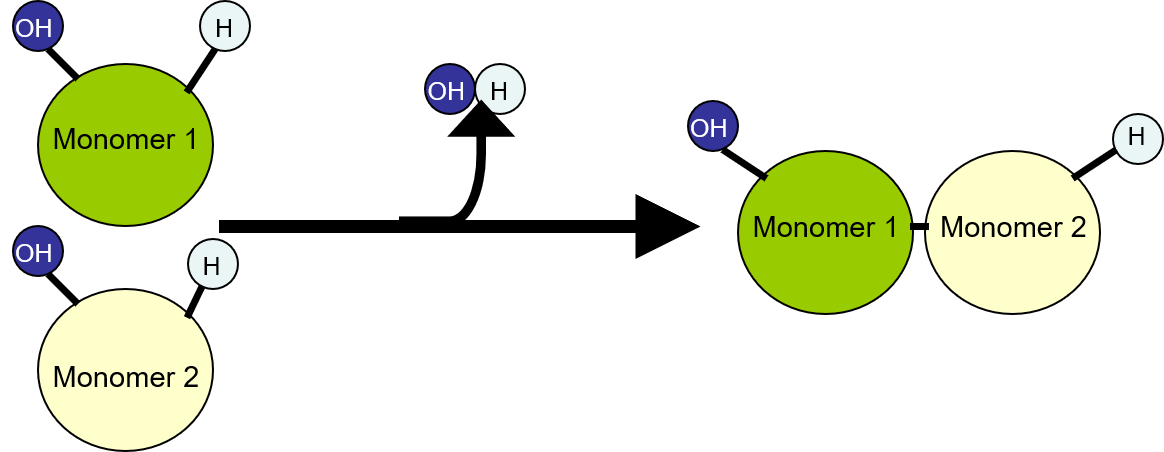 •Carbohydrate metabolism begins with digestion in the small intestine where monosaccharides are absorbed into the blood stream.
•Carbohydrate metabolism begins with digestion in the small intestine where monosaccharides are absorbed into the blood stream.
•Blood sugar concentrations are controlled by hormones. If the concentration of glucose in the blood is too high, insulin is secreted by the pancreas.
•Insulin stimulates the transfer of glucose into the cells, especially in the liver and muscles.
•In the liver and muscles, most of the glucose is changed into glycogen.
•Glycogen is stored in the liver and muscles until needed at some later time when glucose levels are low.
•If blood glucose levels are low, then epinephrine and glucagon hormones are secreted to stimulate the conversion of glycogen to glucose.
•If glucose is needed immediately upon entering the cells to supply energy, it begins the metabolic process.
\n
\n \n \n
\n