Chapter 24: The Innate and Adaptive Immune Systems
Key Terms:
- Pathogens: Causes infection in all organisms. eg: bacteria, viruses, or fungi.
- Restriction factors: Bacteria defend themselves against viruses, using intracellular proteins called restriction factors.
- Innate Immune response: General defense actions that can involve almost any cell type in an organism.
- Adaptive immune response: These are highly specific to the particular pathogen that induced them. these reactions are more sophisticated and specific reactions.
- Lymphocytes: a class of white blood cells.
- two types:
- B Lymphocytes: which secrete antibodies that bind specifically to the pathogen.
- T lymphocytes: which can directly kill cells infected with the pathogen.
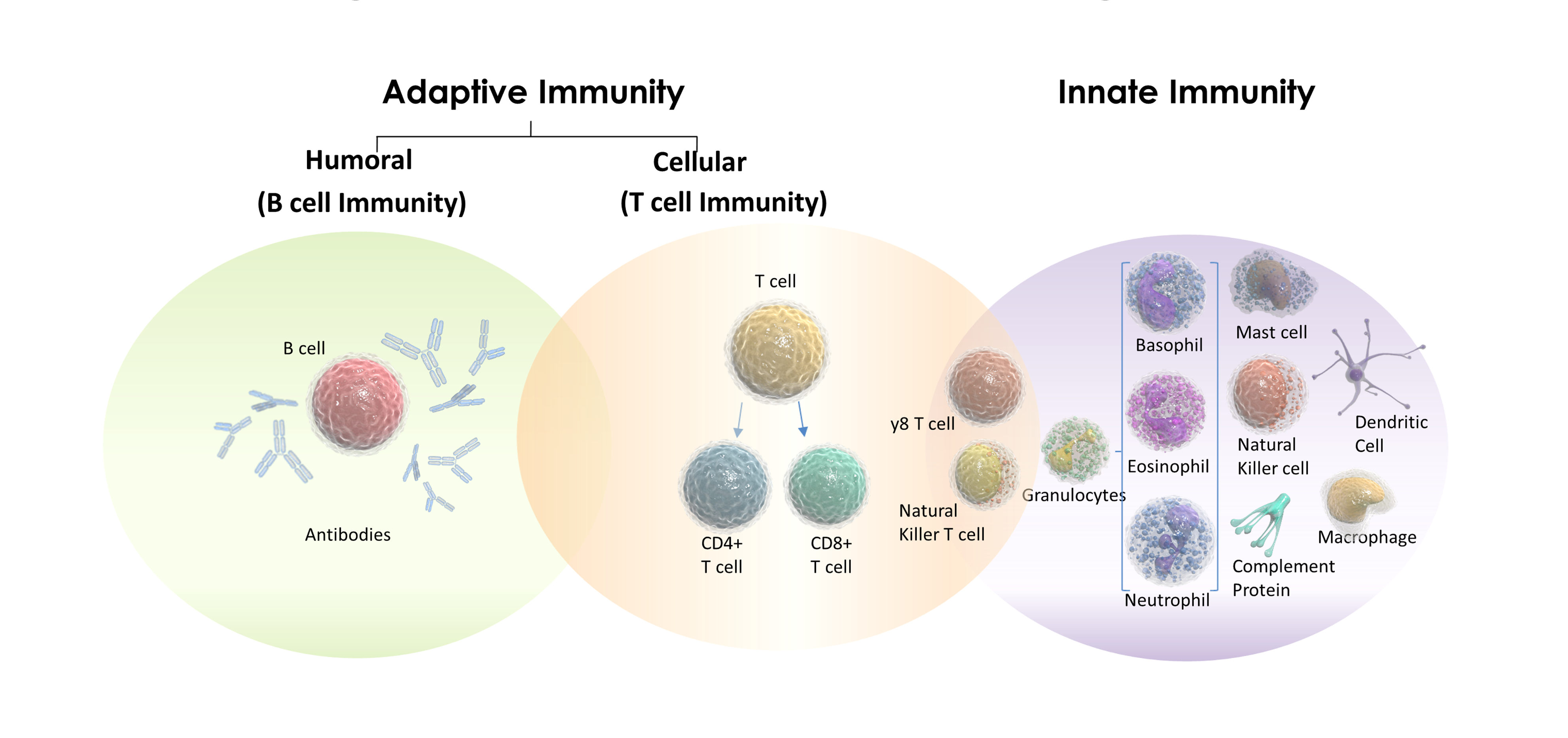
THE INNATE IMMUNE SYSTEM
- It defends against the infection during the first critical hours/days of exposure.
- The first line of defense.
- Plants and invertebrates lack adaptive immune systems and therefore solely rely on the innate immune system.
- Strategies the innate immune system uses to recognize pathogens:
- Epithelial surfaces serve as barriers to infection:
- First encounters are generally the epithelial surfaces that form skin and line the respiratory, digestive and urinary, and reproductive tracts.
- Epithelia provides both physical and chemical barriers to invasion by pathogens.
- Plants and invertebrates secrete, antimicrobial molecules called defensins.
- Pattern Recognition Receptors (PRRs):
- Some pathogens just breach the epithelial surfaces to enter due to some repeating patterns called pathogen-associated molecular patterns. (PAMPs).
- The receptor proteins which recognize PAMPs are called pattern recognition receptors (PRRs).
- PRRs are transmembrane proteins on the surface, where they recognize extracellular pathogens on professional phagocytic cells.
- Multiple Classes of PRRs:
- First identified was the Toll receptor in Drosophila, well-known for its role in fly development.
- TLRs basically toll-like receptors function as PRRs in the innate immune response.
- NOD-like receptors (NLRs) have leucine-rich motifs, they are exclusively cytoplasmic and recognize a distinct set of bacterial molecules.
- Activated PRRs trigger an inflammatory response at sites of infection:
- Pathogen invades a tissue that activates PRRs, resulting in an inflammatory response at the site of infection.
- Activation of PRRs produces a large variety of extracellular signal molecules that mediate the inflammatory response like prostaglandins, cytokines, etc.
- Phagocytic cells seek, engulf, and destroy pathogens:
- The recognition of a microbial invader is usually quickly followed by its engulfment by a phagocytic cell.
- PAMPs activate the macrophage to secrete pro-inflammatory signal molecules.
- PRRs, macrophage, and neutrophils (short-lived phagocytes) display a variety of cell-surface receptors that leads to the binding of the pathogen to these receptors which lead to phagocytosis.
- Phagocytes possess an impressive armory of weapons to kill the invader, including enzymes such as lysozyme, and NADPH oxidase complexes.
Complement activation targets pathogens for phagocytosis or lysis:
- Components of the complement system consist of 30 interacting soluble proteins.
- The early complement components consist of three sets of proteins, belonging to three distinct pathways:
- The classical pathway
- The lectin pathway
- The alternative pathway
- The early components of all three pathways cleave and activate C3.
- The large fragment of C3 called C3b binds covalently to the surface of the pathogen.
- C3b binding receptors on phagocytic cells enhance the ability of these cells to phagocytose the pathogen, and similar receptors on B cells enhance these cells to make antibodies against microbial molecules.
THE ADAPTIVE IMMUNE SYSTEM
The adaptive immune system is one of the two main branches of the immune system, the other being the innate immune system.
It is a complex and highly specialized defense mechanism that provides long-term protection against specific pathogens.
The adaptive immune system is characterized by its ability to recognize, remember, and respond to specific antigens, which are foreign substances such as bacteria, viruses, or toxins.
- This specificity is crucial for targeting and eliminating particular pathogens.
The key players in the adaptive immune system are lymphocytes, a type of white blood cell.
There are two main types of lymphocytes involved: B cells and T cells.
- B cells are responsible for producing antibodies, which are proteins that can bind to specific antigens. Each B cell has a unique receptor on its surface that allows it to recognize and bind to a specific antigen. When a B cell encounters its matching antigen, it is activated and undergoes clonal expansion, producing numerous identical cells that secrete antibodies. These antibodies can neutralize the antigens, mark them for destruction by other immune cells, or activate other components of the immune system.
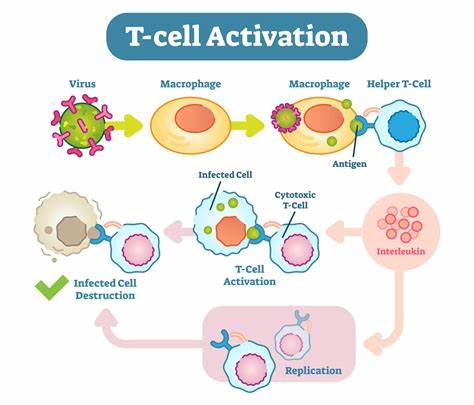
- T cells, on the other hand, have different roles in the adaptive immune response. There are two main types of T cells: helper T cells (CD4+) and cytotoxic T cells (CD8+).
Helper T cells play a central role in coordinating the immune response. They recognize antigens presented on specialized antigen-presenting cells and release chemical messengers called cytokines. These cytokines stimulate and recruit other immune cells, enhance antibody production by B cells, and activate cytotoxic T cells.
Cytotoxic T cells are primarily responsible for identifying and eliminating infected or abnormal cells. They recognize antigens presented on the surface of infected cells and release toxic substances, such as perforin and granzymes, to induce cell death in the target cells.
Both B cells and T cells undergo a process called clonal selection, which ensures that only the lymphocytes capable of recognizing the specific antigen are activated. This process involves the proliferation of specific lymphocytes, leading to the formation of an army of cells that can effectively combat the invading pathogen.
One of the most remarkable features of the adaptive immune system is its ability to form immunological memory. Once an adaptive immune response is initiated, a subset of B and T cells, known as memory cells, are generated. These cells have a long lifespan and retain the ability to recognize the specific antigen encountered during the initial response. In subsequent encounters with the same pathogen, memory cells can mount a faster and more robust immune response, leading to more efficient pathogen clearance.
The adaptive immune system is highly specific, diverse, and adaptable. It can recognize a vast array of antigens, ranging from common pathogens to novel and rapidly evolving ones. This diversity is achieved through genetic recombination and mutation processes that generate an extensive repertoire of B and T cell receptors.
Overall, the adaptive immune system plays a crucial role in defending the body against infections, preventing reinfections, and providing long-term immunity. Its ability to mount targeted and long-lasting immune responses is essential for maintaining health and overcoming various diseases.
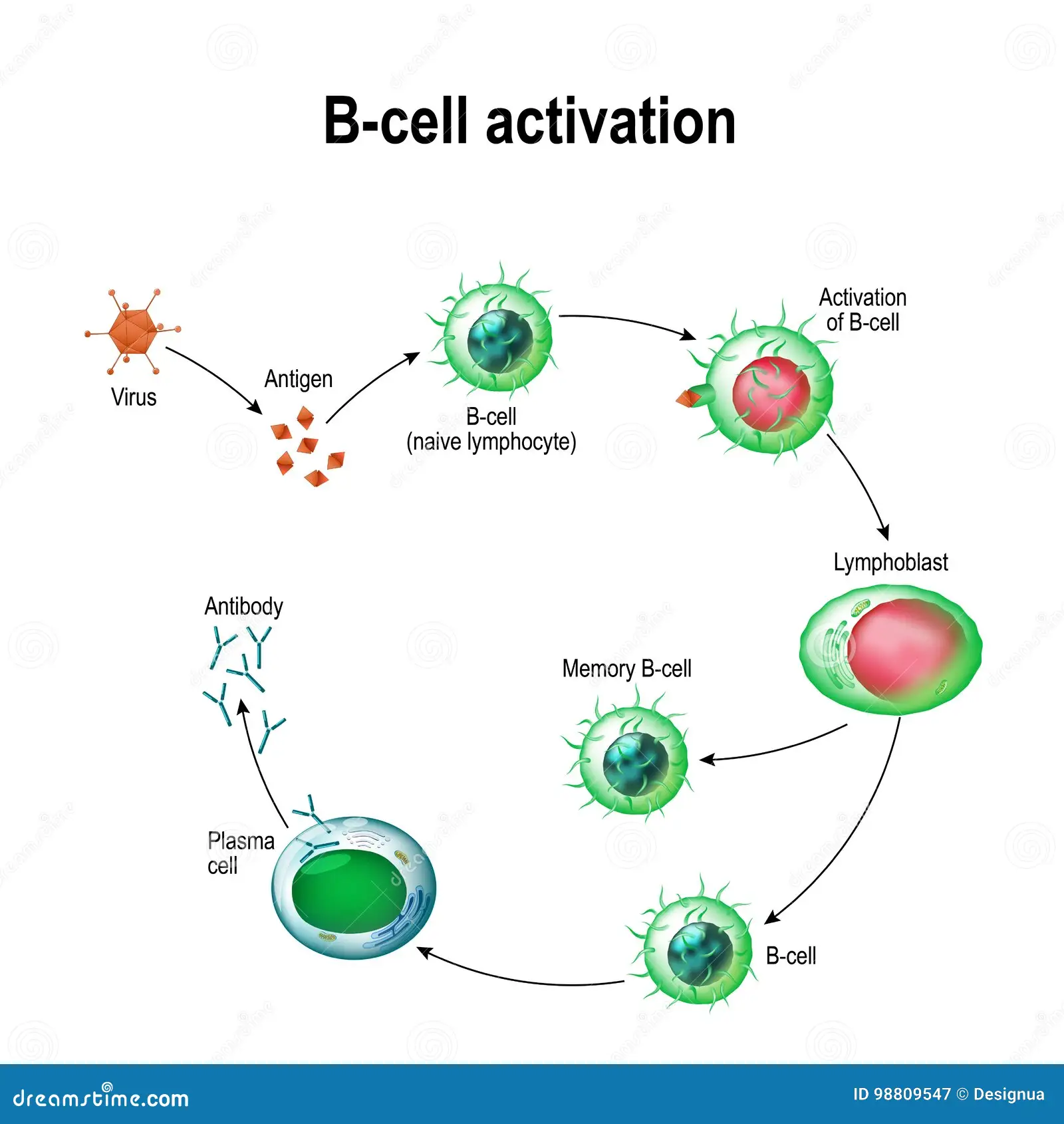
B cells and Immunoglobulins
B cells:
B cells are a type of lymphocyte, a subset of white blood cells, and are an integral part of the adaptive immune system.
B cells develop and mature in the bone marrow.
Each B cell expresses a unique B cell receptor (BCR) on its surface, which consists of membrane-bound immunoglobulins.
The BCR serves as the antigen receptor for B cells, allowing them to recognize specific antigens.
B cells can recognize a wide range of antigens, including proteins, carbohydrates, lipids, and nucleic acids.
When a B cell encounters its specific antigen, it undergoes a process known as activation.
The activation of B cells requires the binding of the antigen to the BCR, along with additional signals from helper T cells.
Once activated, B cells undergo clonal expansion, leading to the production of a large number of identical B cells called plasma cells.
Plasma cells are specialized for antibody production and secrete large amounts of soluble antibodies.
In addition to plasma cells, a subset of activated B cells differentiates into memory B cells.
Memory B cells have a longer lifespan compared to plasma cells and can provide a rapid and enhanced immune response upon re-exposure to the same antigen.
B cells play a crucial role in humoral immunity, which involves the production and secretion of antibodies.
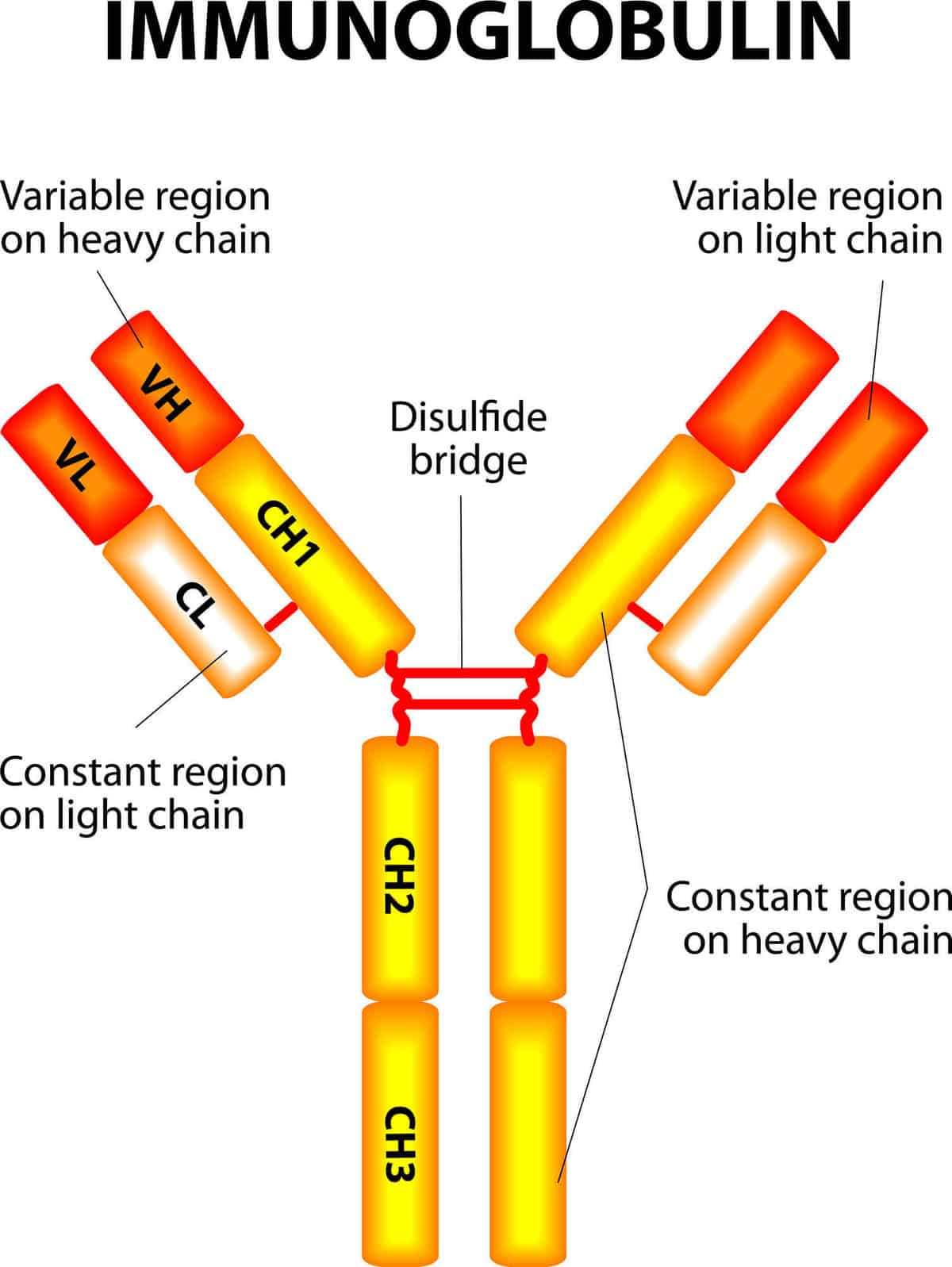
Immunoglobulins:
Immunoglobulins, also known as antibodies, are Y-shaped proteins produced by B cells and plasma cells.
They are part of the larger family of glycoproteins called immunoglobulin superfamily.
Each immunoglobulin molecule consists of two heavy chains and two light chains, held together by disulfide bonds.
There are five major classes of immunoglobulins: IgM, IgG, IgA, IgE, and IgD.
- IgM is the first antibody produced during an immune response and is particularly effective at activating the complement system.
- IgG is the most abundant class of antibodies in the bloodstream and is involved in long-term immunity.
- IgA is primarily found in secretions such as saliva, tears, and breast milk, providing localized protection on mucosal surfaces.
- IgE is involved in allergic reactions and defense against parasitic infections.
- IgD is primarily found on the surface of naive B cells, serving as a BCR.
Each immunoglobulin class has distinct properties and functions, allowing for a diverse range of immune responses.
Immunoglobulins function by binding to antigens with high specificity through their antigen-binding sites, located at the tips of the Y-shaped molecule.
Upon antigen binding, immunoglobulins can neutralize pathogens, mark them for destruction by other immune cells, activate complement proteins, and enhance phagocytosis.
Immunoglobulins can also participate in allergic reactions by binding to allergens and triggering the release of histamine from mast cells.
The structure and function of immunoglobulins are highly adaptable, allowing for the generation of a vast repertoire of antibodies capable of recognizing a wide range of antigens.
T cells and MHC Proteins
T cell
T cells are a type of lymphocyte, a subset of white blood cells, and are key players in the adaptive immune system.
T cells develop in the bone marrow from hematopoietic stem cells but undergo maturation in the thymus gland, hence the name "T" cells.
The main function of T cells is to recognize specific antigens and coordinate immune responses.
T cells express a unique T cell receptor (TCR) on their surface, which recognizes specific antigens presented by major histocompatibility complex (MHC) molecules on the surface of other cells.
There are two main types of T cells: helper T cells (CD4+) and cytotoxic T cells (CD8+).
- Helper T cells play a central role in regulating immune responses. They recognize antigens presented by antigen-presenting cells (APCs) and provide crucial signals to activate and coordinate other immune cells.
- Helper T cells release chemical messengers called cytokines, which stimulate immune responses, recruit other immune cells to the site of infection, and enhance the activity of B cells, cytotoxic T cells, and phagocytes.
- Cytotoxic T cells are primarily responsible for eliminating infected or abnormal cells. They recognize antigens presented on the surface of infected cells or cancer cells, directly interacting with them to induce cell death.
- Cytotoxic T cells release cytotoxic substances, such as perforin and granzymes, which create pores in the target cell's membrane and trigger programmed cell death.
- Another subset of T cells called regulatory T cells (Tregs) plays a critical role in immune tolerance and preventing excessive immune responses. Tregs help maintain immune homeostasis by suppressing the activity of other immune cells and preventing autoimmunity.
T cells also exhibit a process known as clonal selection, similar to B cells. When a T cell encounters its specific antigen, it undergoes activation and clonal expansion to generate a population of antigen-specific T cells.
Memory T cells, similar to memory B cells, are long-lived T cells that persist after an initial immune response. They provide rapid and enhanced immune responses upon re-exposure to the same antigen, contributing to immunological memory.
T cells are involved in a wide range of immune responses, including defense against viral infections, intracellular pathogens, cancer cells, and regulation of immune responses to prevent autoimmune diseases.
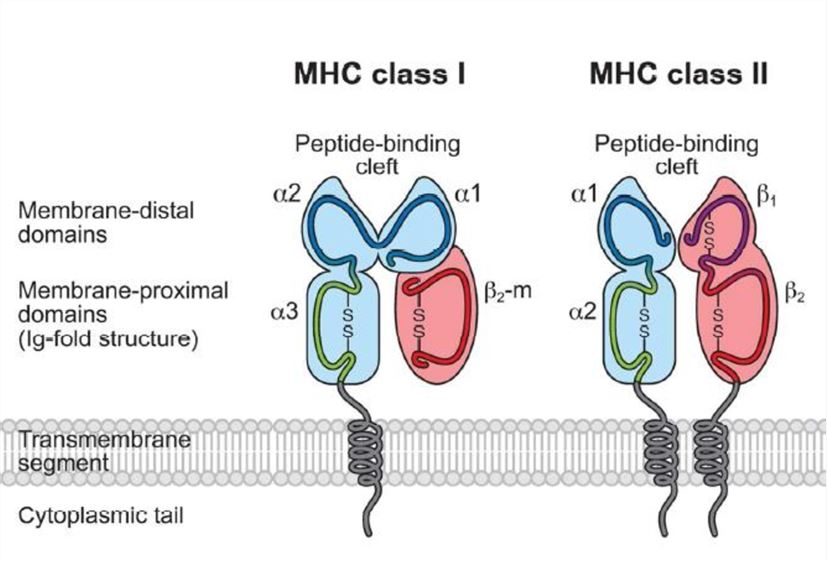
MHC proteins
MHC proteins, also known as human leukocyte antigens (HLAs) in humans, are cell surface molecules that present antigens to T cells.
MHC proteins are divided into two main classes: MHC class I and MHC class II.
- MHC class I molecules are found on the surface of most nucleated cells and present antigens derived from intracellular pathogens, such as viruses or intracellular bacteria.
- MHC class I molecules consist of a heavy chain and a small protein called beta-2 microglobulin. They bind to short peptide fragments (8-10 amino acids) derived from intracellular proteins.
The peptide binding groove of MHC class I molecules is closed at both ends, allowing them to bind peptides with a specific sequence and anchor residues at specific positions.
MHC class I molecules present antigens to cytotoxic T cells (CD8+ T cells). Cytotoxic T cells recognize the complex of MHC class I molecule and antigen peptide on infected or abnormal cells, leading to the elimination of those cells.
MHC class II molecules are primarily found on the surface of antigen-presenting cells (APCs), such as dendritic cells, macrophages, and B cells.
- MHC class II molecules consist of two chains, alpha and beta, both of which are encoded by genes within the MHC region.
- MHC class II molecules have an open peptide binding groove that can accommodate longer antigen peptides (typically 13-25 amino acids) derived from extracellular pathogens.
APCs capture antigens from the extracellular environment, process them within intracellular compartments, and present antigen peptides bound to MHC class II molecules on their surface.
MHC class II molecules present antigens to helper T cells (CD4+ T cells). Helper T cells recognize the complex of MHC class II molecule and antigen peptide, triggering immune responses and coordinating the activity of other immune cells.
The binding specificity of MHC molecules is determined by polymorphic residues in the peptide-binding groove, which can accommodate a diverse range of antigen peptides.
MHC molecules are highly polymorphic, meaning that there are multiple allelic variants within a population. This polymorphism contributes to the diversity of antigens that can be presented and recognized by T cells.
The interaction between T cell receptors (TCRs) on T cells and the MHC-antigen complex is highly specific. Each TCR recognizes a particular antigen peptide in the context of a specific MHC molecule.
This specific recognition allows T cells to discriminate between self and non-self antigens, ensuring an appropriate immune response.
MHC restriction refers to the requirement of T cells to recognize antigens only when presented in association with the appropriate MHC molecule.
MHC proteins play a crucial role in the adaptive immune response by facilitating the recognition of antigens and initiating T cell activation and coordination of immune responses.