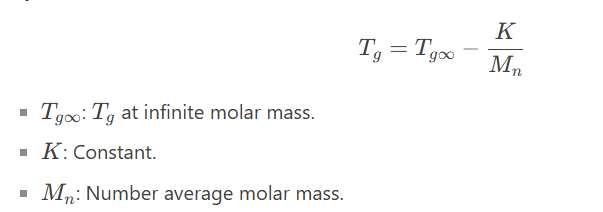L5: The Amorphous State of Polymers and Glass Transition Temperature
The Amorphous State of Polymers
Definition: Amorphous polymers lack long-range order (i.e., they are non-crystalline).
Characteristics:
No diffraction patterns (unlike crystals).
Exhibits a glass transition temperature (Tg).
Below Tg: Polymer is in a glassy state (rigid, brittle).
Above Tg: Polymer is in a rubbery state (flexible, chain mobility increases).
Examples: Atactic polystyrene, atactic PMMA.
2. Glass Transition Temperature (Tg)
Definition: Tg is the temperature at which an amorphous polymer transitions from a glassy to a rubbery state.
Thermodynamic Nature:
Second-order transition-like: Discontinuity in the rate of change of properties (e.g., specific volume, heat capacity).
Not a true second-order transition: Tg depends on the cooling rate.
Key Changes at Tg:
Heat capacity (Cp), thermal expansion coefficient (α), and compressibility (κ) change.
Free volume (Vf): The extra space created by molecular vibrations increases above Tg.
3. Free Volume and Tg
Free Volume (Vf): The space available for molecular motion, created by thermal vibrations.
Equation:
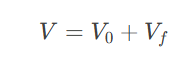
V: Total volume.
V0: Occupied volume.
Vf: Free volume.
Fractional Free Volume (fv):
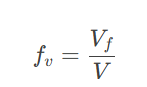
At Tg: Free volume reaches a critical value, freezing the polymer chains in place.
4. Factors Affecting Tg
1. Chain Flexibility:
More flexible chains: Lower Tg (e.g., poly(dimethylsiloxane) has Tg≈−120∘C).
Rigid chains: Higher Tg (e.g., poly(phenylene sulfone) has no Tg).
2. Steric Hindrance:
Bulky side groups: Increase Tg by restricting chain rotation (e.g., polystyrene Tg≈100∘C).
Flexible side groups: Decrease Tg (e.g., poly(methyl acrylate) Tg≈10∘C.
3. Branching:
Low branching: Decreases Tg (increases free volume).
High branching: Increases Tg (restricts chain mobility).
4. Crosslinking:
Increases Tg: Reduces free volume and restricts chain mobility.
5. Polymer Molar Mass (MM):
Higher MM: Increases Tg (reduces chain mobility).
Equation:
6. Additives (Plasticizers):
Lower Tg: Increase free volume and chain mobility (e.g., plasticizers in PVC reduce Tg for flexibility).
7. Chemical Composition:
Miscible blends: Show a single Tg between the Tg values of the components.
Immiscible blends: Show separate Tg values for each component.
5. Thermodynamic Theory of Tg (Gibbs-di Marzio Theory)
T2: A theoretical second-order transition temperature where the configurational entropy of the system is zero.
T2 is approximately 50 K below Tg.
6. Practical Implications of Tg
Thermoplastics: Soften above Tg and can be reshaped (e.g., polypropylene, PMMA).
Thermosets: Permanently harden upon heating due to crosslinking (e.g., epoxy resins).
Elastomers: Exhibit large reversible deformations above Tg (e.g., natural rubber).
7. Summary of Key Points
Amorphous polymers lack long-range order and exhibit a glass transition temperature (Tg).
Tg is the temperature at which the polymer transitions from a glassy to a rubbery state.
Free volume plays a critical role in determining Tg, with molecular motion increasing above Tg.
Factors affecting Tg: Chain flexibility, steric hindrance, branching, crosslinking, molar mass, additives, and chemical composition.
Thermodynamic theory: Tg is influenced by configurational entropy and free volume.
Learning Outcomes
By the end of this lecture, you should be able to:
Understand the amorphous state of polymers and how it differs from the crystalline state.
Define the glass transition temperature (Tg) and explain its thermodynamic nature.
Explain the concept of free volume and its role in determining Tg.
Describe the factors that affect Tg, including chain flexibility, steric hindrance, branching, crosslinking, molar mass, and additives.
Calculate Tg using the relationship between Tg and molar mass.
Understand the practical implications of Tg for different types of polymers (thermoplastics, thermosets, elastomers).
Apply Gibbs-di Marzio theory to understand the thermodynamic basis of Tg
 Knowt
Knowt