
water
structure
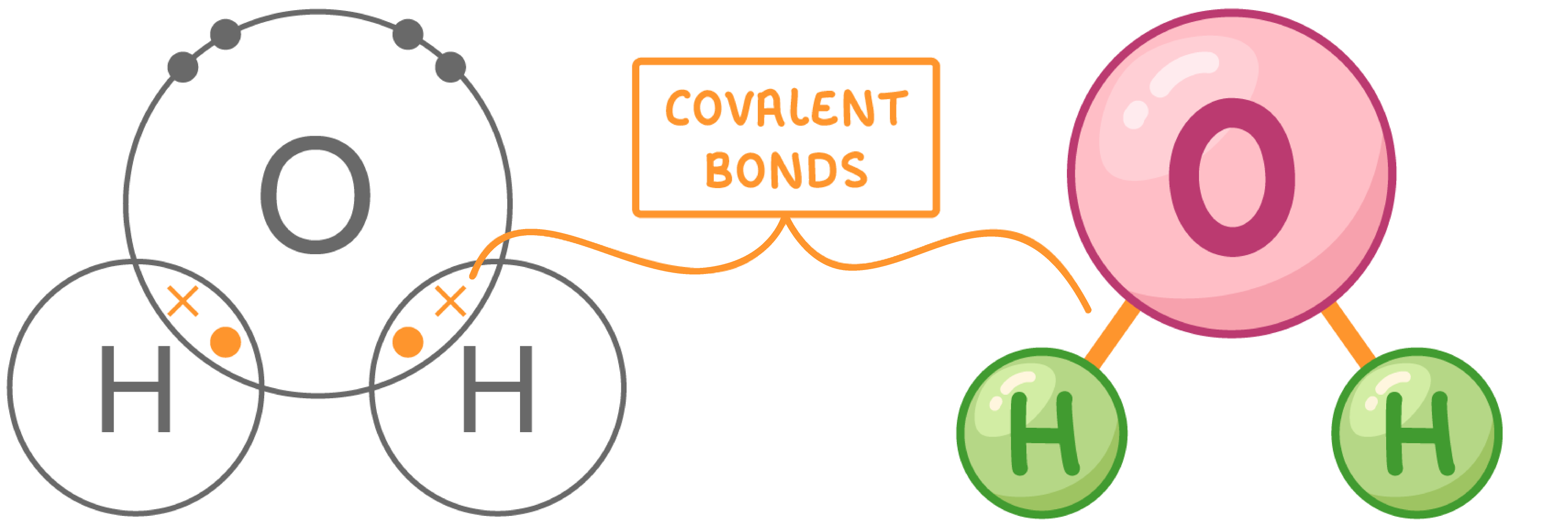
(di)polar molecule → solvent (where reactions take place)
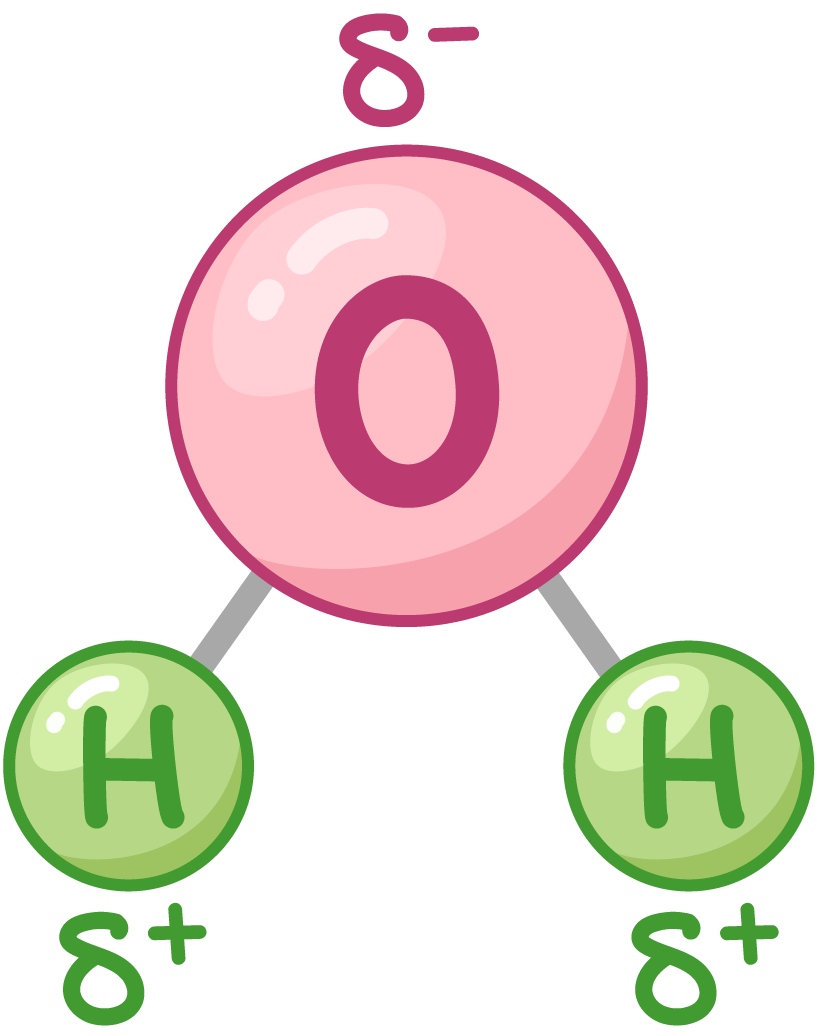
shared electrons pulled to O atom → slightly neg charge. H atom → slightly pos charge
both pos + neg poles → dipolar
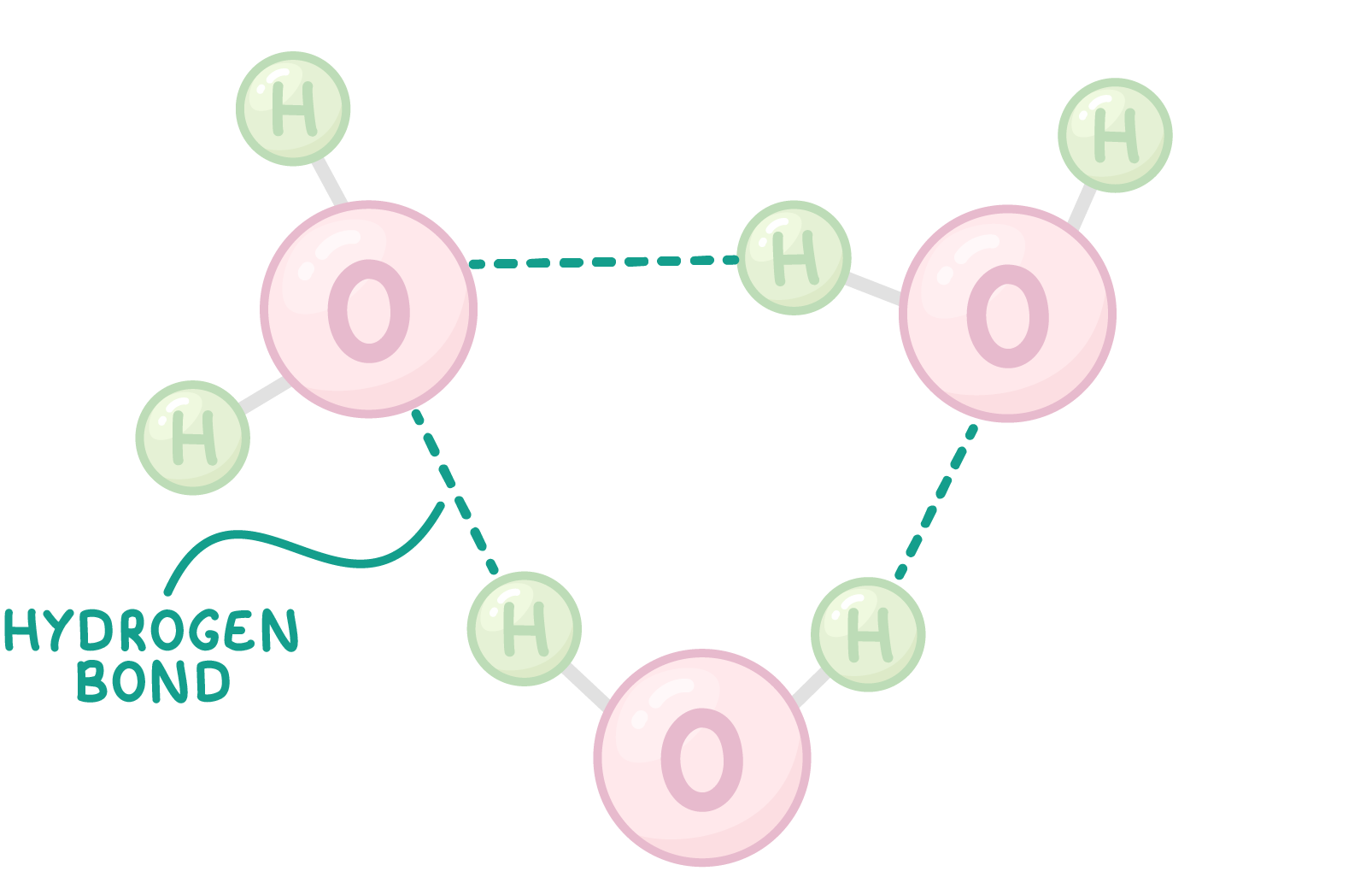
water molecules can form H bonds
δ- O end and δ+ H end → water molecules interact w each other.
hydrogen bond: δ+ H end of one water molecule attracts towards δ+ H end of another molecule
roles of water
solvent - lots substances dissolve in water
ionic compounds (made of pos+neg ions) split apart when added to water.
buffer temp fluctuations - makes/maintains stable conditions (reactions). high SHC bc of H bond. more stable = less energy. bonded = less energy
cooling mechanism/sweating - large latent heat, more H bonds needed to break
habitat - organisms survive/reproduce in water
metabolite in condensation + hydrolysis reactions
transport - organisms use to move substances
water as solvent
many substances within cells are ionic compounds, means they consist of positive + negative ions (e.g. salt made up of Na+ + Cl- ions). When these ionic compounds are added to water, ions are split apart

water is polar so slightly neg oxygens attracted to pos ions whilst slightly pos hydrogens attracted to neg ions
each ion surrounded by water molecules + compound dissolves
water known as universal solvent bc it dissolves more substances than any other liquid
useful for following reasons:
most biological reactions take place in solution - ex in cytoplasm of eukaryotes/prokaryotes
dissolved substances can be transported around body - ex ions can be transported in blood plasma
water as temp buffer
has high specific heat capacity, requiring much energy to raise 1g of water by 1°C.
Hydrogen bonds absorb energy, making them hard to break + heat water.
High SHC makes water resist rapid temp changes.
Organisms made of water maintain stable body temp
SHC can be defined per 1g or 1kg, depending on units used in calculations
water as cooling mechanism
Hydrogen bonding requires much energy to evaporate 1g of water.
has high latent heat of vaporization, needing signif energy to turn from liquid → gas.
Organisms use water evaporation for cooling w out losing much water.
evaporation from skin takes heat energy away, cooling organism
water as habitat
high SHC and LHV create stable environment.
water freezes → less dense ice, floats.
floating ice insulates ponds + lakes
water below ice stays unfrozen, allowing organisms to survive
water as metabolite
involved in many chemical reactions in organisms:
Hydrolysis: uses water to break down molecules
Condensation: releases water to join molecules
Photosynthesis: uses water as raw material
water as transport medium
Cohesion: Water molecules stick together via hydrogen bonds.
Adhesion: Water sticks to other materials.
Cohesion and adhesion help water flow through organisms, transporting substances.

In plants, cohesion and adhesion allow water to move through the xylem.
Water's high surface tension creates skin-like surface that supports small organisms like pond-skaters.
water
structure

(di)polar molecule → solvent (where reactions take place)
shared electrons pulled to O atom → slightly neg charge. H atom → slightly pos charge
both pos + neg poles → dipolar

water molecules can form H bonds
δ- O end and δ+ H end → water molecules interact w each other.
hydrogen bond: δ+ H end of one water molecule attracts towards δ+ H end of another molecule

roles of water
solvent - lots substances dissolve in water
ionic compounds (made of pos+neg ions) split apart when added to water.
buffer temp fluctuations - makes/maintains stable conditions (reactions). high SHC bc of H bond. more stable = less energy. bonded = less energy
cooling mechanism/sweating - large latent heat, more H bonds needed to break
habitat - organisms survive/reproduce in water
metabolite in condensation + hydrolysis reactions
transport - organisms use to move substances
water as solvent
many substances within cells are ionic compounds, means they consist of positive + negative ions (e.g. salt made up of Na+ + Cl- ions). When these ionic compounds are added to water, ions are split apart

water is polar so slightly neg oxygens attracted to pos ions whilst slightly pos hydrogens attracted to neg ions
each ion surrounded by water molecules + compound dissolves
water known as universal solvent bc it dissolves more substances than any other liquid
useful for following reasons:
most biological reactions take place in solution - ex in cytoplasm of eukaryotes/prokaryotes
dissolved substances can be transported around body - ex ions can be transported in blood plasma
water as temp buffer
has high specific heat capacity, requiring much energy to raise 1g of water by 1°C.
Hydrogen bonds absorb energy, making them hard to break + heat water.
High SHC makes water resist rapid temp changes.
Organisms made of water maintain stable body temp
SHC can be defined per 1g or 1kg, depending on units used in calculations
water as cooling mechanism
Hydrogen bonding requires much energy to evaporate 1g of water.
has high latent heat of vaporization, needing signif energy to turn from liquid → gas.
Organisms use water evaporation for cooling w out losing much water.
evaporation from skin takes heat energy away, cooling organism
water as habitat
high SHC and LHV create stable environment.
water freezes → less dense ice, floats.
floating ice insulates ponds + lakes
water below ice stays unfrozen, allowing organisms to survive
water as metabolite
involved in many chemical reactions in organisms:
Hydrolysis: uses water to break down molecules
Condensation: releases water to join molecules
Photosynthesis: uses water as raw material
water as transport medium
Cohesion: Water molecules stick together via hydrogen bonds.
Adhesion: Water sticks to other materials.
Cohesion and adhesion help water flow through organisms, transporting substances.

In plants, cohesion and adhesion allow water to move through the xylem.
Water's high surface tension creates skin-like surface that supports small organisms like pond-skaters.
 Knowt
Knowt