Chemistry Final Exam Study Guide
Gas Laws
The 5 natures of gasses:
Gasses have mass (low density compared to solids and liquids)
Gasses are compressible
Gasses fill their containers
Gasses diffuse (move from areas of high to low concentration)
Size & energy influence speed of diffusion (heavier atomic mass -> slower diffusion)
Gasses exert pressure (sum of collisions of gas molecules with themselves and their container)
Atmospheric pressure: Pressure of gasses in the air, varies with altitude
Measured with a barometer
Definition and units of the following (conversion factors will be provided):
Pressure: 1 atm = 760 torr = 760 mmHg = 101.3 kPa
Temperature: 0ºC = 273.15 K
Volume: 1 L = 1000 mL = 1000 cm^3
Standard temperature and pressure (STP): 0ºC & 1 atm (freezing point of water at sea level)
The gas laws (MUST USE KELVIN!!!)
Boyles: P1*V1 = P2*V2 (pressure and volume are inversely proportional)
Charles: V1/T1 = V2/T2 (Volume and temp are directly proportional)
Gay Lussac's: P1/T1 = P2/T2 (pressure and temp are directly proportional)
Combined: P1*V1/T1 = P2*V2/T2
Dalton’s law of partial pressure: Ptotal = P1 +P2 + P3…
Ideal: PV = nRT (number of moles (n) of contained gas)
International gas law constant (R): Changes depending on unit of pressure
R for atm = 0.0821
R for mmHg and torr = 62.4
R for kPa = 8.314
Manometer: Tool used to measure the pressure of contained gas by comparing it to atmospheric pressure
Manometer problems (P = pressure):
If Pgas > Patm: Pgas = Patm + ∆z
If Pgas < Patm: Pgas = Patm - ∆z
Gas stoichiometry
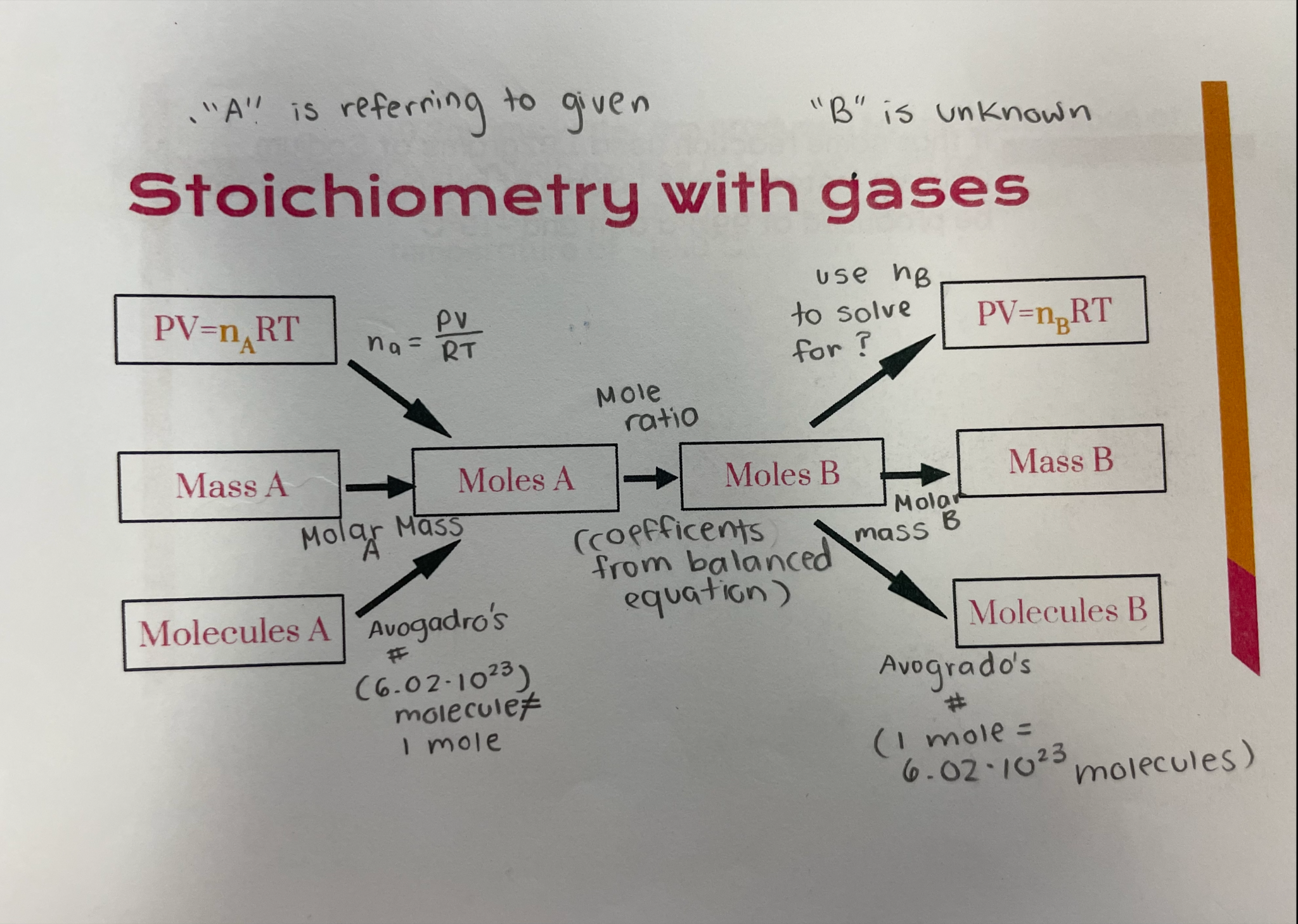
Use mole ratio when solving for
Molar mass
Na -> Nb
# molecules a -> # molecules b
Avigadro’s number in stoichiometry
1 mole (6.02*10^23) = the amount of molecules in an element as appears on periodic table
Anything from the lab with all of the stations where we explored real world concepts
Solutions Concepts
All things water
Structure: Water is a polar molecule because it has an uneven distribution of electrons
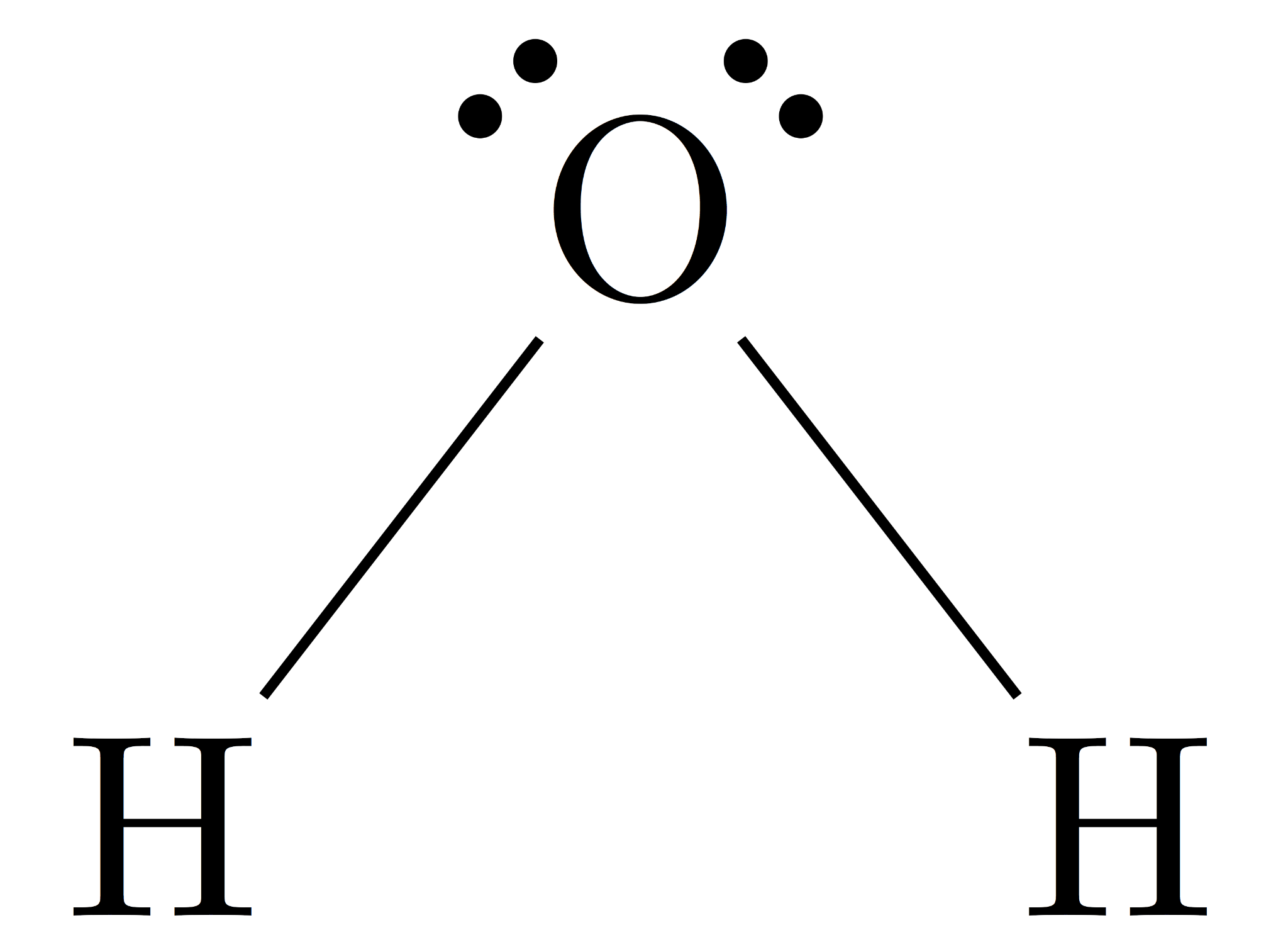
4 unusual properties and why
High surface tension: Water is polar & very attracted to itself via h-bonding which creates a sort of skin on the surface of the water
High boiling point: Water is polar & very attracted to itself via h-bonding, so it takes lots of energy for H2O to break h-bonds and escape as vapor
Ice is less dense than water: Frozen water forms a lattice pattern which fills with air and causes ice to be less dense than water
Water is the universal solvent: Because water is polar, it has the ability to attract other polar molecules and pull them apart
“Like dissolves like”: Polar dissolves polar, nonpolar dissolves nonpolar
Cohesion: ability of a molecule to stick to itself
Adhesion: ability of a molecule to stick to something else
Solution formation
Hydration: Solvation where water is the solvent
Solvation: Word for dissolving
Solute vs solvent: Solute is being dissolved, solvent is doing the dissolving
Factors affecting rate of solvation and why:
Stirring: makes H2O molecules tear the solute apart
Temperature: increased temp -> increased KE -> increased collisions and contact between solute and solvent
Particle Size: Decreased particle size -> increased surface area and contact
Solubility: amount of solute that can dissolve at given temp (g solute/100 g solvent)
Factors affecting solubility:
Gasses
Temperature is directly proportional to solubility
Increase in temp -> increase in solubility
Pressure: N/A
Solids
Temperature is inversely proportional to solubility
Increase in temp -> decrease in solubility
Pressure is directly proportional to solubility
Increase in pressure -> increase in solubility
Saturation: number of solute particles that are dissolved in a solvent at a given temperature
Saturated solution: max amount of solute dissolved at given temp
Unsaturated: less solute that can theoretically be dissolved at given temp
Supersaturated: through a process, more solute dissolved than theoretically possible
Measurements of solution
Concentration: amount of solute in a solution to either solvent or total solution
Dilute solution: small amount of solute
Concentrated solution: max amount of solute
Molarity (M): moles of solute/liters of solution
Molality (m): moles of solute/kilograms of solvent
Intermolecular forces: forces of attraction between molecules due to polarity and movement of electrons within the bond
The fundamental difference between states of matter is the strength if the IMFs holding them together
Types of IMFs:
Ion dipole: Exist between an ionic compound in water (ex: Cl and positive poles in water)
Strongest IMF!!!
Hydrogen bonding: Stronger version of DP-DP, hydrogen with strong positive charge due to electronegativity of O, N, or F becomes attracted to the O, N, or Fs of other molecules
Dipole dipole: Polar molecules with oppositely charged ends attract each other
Medium IMF
London dispersion: exists between all molecules; is a temporary force that exists when adjacent electrons are positioned to make the atoms form temporary dipoles
Is the weakest IMF!!!
Polarity: Uneven distribution of electrons creates positive and negative poles on a bond
How IMFs influence boiling point & melting point: Stronger IMFs -> more energy required for state change -> higher boiling point & lower melting point
If same IMF, increase in mass of molecule -> increase in strength of dispersion force
Colligative properties: properties that depend only on the number of solute particles and not their identity
BP elevation: Difference in temp of boiling point of solution vs boiling point of pure solvent
FP depression vs FP of solution: Difference in temp between freezing point of solution vs freezing point of pure solvent
Molal freezing point and boiling point constants: depend on solvent
Kf for water is 1.86º C/m
Kb for water is 0.521º C/m
Steps to solve colligative property problems:
Solve for molality
Solve for ∆Tf & ∆Tb
∆Tf = Kf * m
∆Tb = Kb * m
Solve for new boiling or freezing point
BP solution = BP + ∆Tb (elevation)
FP solution = FP + ∆Tf (depression)
Rate and Equilibrium
Collision theory: Atoms, ions, and molecules with sufficient energy can react to form products when they collide
Activation energy: The minimum energy needed by colliding particles in order to react
Activated complex: An unstable arrangement of atoms that exists momentarily at the peak of the activation energy barrier
Reaction rate:
What is it?
4 factors affecting it
Concentration: More particles -> more collisions -> faster rate
Temperature: Increase in temp -> more collisions -> faster rate
Particle Size: Increase in surface area -> more collisions -> faster rate
Catalyst: Makes reaction easier by lowering activation energy
The rate law: shows the relationship of the reaction rate to the rate constant and the concentrations of the reactants raised to some power
For aA + bB -> cC + dD:
Rate = k[A]^x[B]^y
How to solve for rate law equations
How to determine the order of a reactant
What is “overall order”: reaction is (x+y)th order overall
How to set up rate law expression
How to solve for rate law constant
Reversible reaction
Forward reaction
Reverse reaction
Equilibrium: when the rate of the forward and reverse reactions are equal and the concentrations of reactants and products don’t change
Le chatlier’s principle: If stress is applied to a system (reaction) at equilibrium, the system changes to relieve the stress
What does it mean
The stresses that exist
Change in concentration: If you remove a reactant or product, the reaction will favor the reaction making it (opposite also true)
Change in temperature: If you heat a system it will favor the reaction using heat (opposite also true)
Exothermic: absorb heat (heat is a reactant)
Endothermic: give of heat (heat is a product)
Change in pressure/volume: If pressure is increased the system will favor the reaction making less moles of gas (opposite also true)
IF NO GASSES, NO EFFECT!!!
Keq expression
How to set it up
What the value tells you
ICE tables: stand for:
Initial concentration
Change in concentration
Equilibrium concentration
They are set up in the following manner:
aA |
| <-> cC | |
|---|---|---|---|
Initial | |||
Change | |||
Equilibrium |
Given Keq
Asked for Keq
Acids and Bases
Properties:
Acids contain an ionizable hydrogen, are sour, and have a pH of less than 7
Bases contain an ionizable hydroxide, are bitter, slippery when wet, and have a pH of more than seven
Both conduct electricity
6 strong acids & bases: Complete ionization in water
6 strong acids | 6 strong bases |
|---|---|
HClO4 (perchloric acid) | LiOH (lithium hydroxide) |
HCl (hydrochloric acid) | AQZNaOH (sodium hydroxide) |
HBr (hydrobromic acid | KOH (potassium hydroxide) |
HI (hydroiodic acid) | Ca(OH)2 (calcium hydroxide) |
HNO3 (nitric acid) | Sr(OH)2 (strontium hydroxide) |
H2SO3 (sulfuric acid) | Ba(OH)2 (barium hydroxide) |
Arrhenius acids and bases
Arrhenius acids give off hydrogen in water
Arrhenius bases give off hydroxide in water
Mono, di, & triprotic acids: Acids with one, two and three ionizable hydrogens
Bronstead lowry acids and bases: Relationship between them that does not involve water
Acids give off hydrogen ions
Bases can gain hydrogen ions
Amphoteric substances ca lose or gain a hydrogen ion
Conjugate acids: The product formed when a base gains a hydrogen
Conjugate bases: The product formed when an acid loses a hydrogen
pH
Ion product constant (Kw): At 25ºC, Kw = 1.0*10^-14
In pure water [H] = 1.0*10^-7
Equations:
If given [H]
pH = -log([H])
[OH] = kw/[H]
If given pH
pOH = 14-pH
[H] = 10 ^-pH
If given [OH]
pOH = -log([OH])
[H] = kw/[OH]
If given pOH
pH = 14-pOH
[0H] = 10 ^-pOH
pH and pOH scale: Tells you concentration of hydrogen
Low pH value = stronger acid
Indicators:
Acid/base indicators: Very specific, ~ 1.5 pH range
Universal indicators: Unspecific, 1-12 pH ranger
Weak acids and bases: Do not fully dissociate (dissolve) in water
Pairing with conj acids & bases: Weak acids -> strong conj bases (opposite also true)
Ka & Kb: Extent of proton transfer between the acid/base and H2O
Determines strength of acid/base (smaller ka = weaker acid)
Ka equation: Keq * [H20] = [H3O][A]/[HA] (products/reactants)
Solving Ka/Kb steps (given: M and pH)
Is it a strong acid? if not, make ICE table
create ICE table & Ka equation
use pH to find [H]
plug in [H] for x in Ka equation
Solving [OH] & pH steps (given Ka/Kb, M)
check to see if [HA]/Kb > 500
create ICE table & Kb equation
use Kb equation to find Kb using algebra
use [OH] to find pH
Ha and Ka relationship:
if [HA]/Kb > 500: change in initial concentration of x is negligible (can remove x from E row)
if [HA]/Kb < 500: change in initial concentration of x is not negligible (must keep x in E row)
% dissociation
Acids: final [H30]/initial acid * 100
Bases: final [OH]/initial base * 100
Titration
Neutralization reactions: when an acid and a base react and neutralize each other (moles H = moles OH, produces water)
Neutralized products formula: combine cation from base and anion from acid + HOH to make product formulas
Neutralization problems: Given V & M of one substance, V of another (use train tracks to solve for M)
Buret: a graduated glass tube with a tap at one end, for delivering known volumes of a liquid, especially in titrations
Titrant/standard solution: The substance of known concentration added to the analyte in a titration
Analyte: The substance of unknown concentration
Equivalence point: Point in a titration where neutralization occurs
End point: Point at which the indicator changes color in titration