Lecture 1: Altered cellular and tissue biology
cells adapt to their environment to protect themselves from injury
an adapted cell is neither normal nor injured
physiologic- the cell may have enhanced function
pathogenic- extreme adaptation to excessive functional demand
the most significant adaptive changes in cells include:
atrophy- decrease or shrinkage in cellular size
e.g. thymus gland undergoes physiologyic atrophy
pathogenic atrophy occurs as a result of decrease in blood supply, pressure, nutrition, workload, hormonal sinulation, and nervous simulation
hypertrophy- increase in cellular size (which leads to increased organ size)
increase in size is due to increased accumulation of protein in cellular components
caused by specific hormone simulation or increased functional demand
hyperplasia- increase in the number of cells (thus increase rate of cellular division)
caused in response to prolonged or severe injury
loss of cells trigger dna synthesis and mitosis
compensatory hyperplasia- enables an organ to regenerate
e.g. liver, even with removal of 70%, regeneration can be comppleted in 2 weeks
hormonal hyperplasia- occurs mainly in estrogen dependent organs (uterus, breast, etc)
metaplasia- the reversible replacement of one mature cell by another
it is as if the original cells are not robust enough to withstand the new environment, and so they change into another type more suited to the new environment
e.g. cigarette smoke that causes the mucus secreting ciliated simple columnar respiratory epithelial cells that line the airways to be replaced by simple squamous epithelium, or a stone in the bile duct that causes the replacement of the secretory columnar epithelium with simple squamous epithelium
caused by reprogramming of stem cells
dysplasia- abnormal change in the size, shape, and organization of mature cell
cell cycle and possible sites of block:
m phase → (g0 phase) [not always done, not always exited] → g1 phase → s phase → g2 phase → m phase …
between m and g0 there is a possible site of block leading to cell hyperplasia
permanently nondividing cells (neurons, normoblasts, adult myocardial cells etc) will leave the cell cycle between m and g0
the cells of the liver and kidney will go to g0 and can leave it if stimulated by subtotal hepatectomy, renal tubular necrosis, nephrectomy etc.
between s and g2 there is a possible site of block leading to cell hypertrophy
most disease begins with cell injury
injury occurs if the cell is unable to maintain homeostasis
injured cells may be able to recover but may not (reversible vs irreversible injury)
major disturbances and damage to the membrane or lack of atp generation due to mitochondrial dysfunction
injurious stimuli could be:
chemical agent
hypoxia (lack of oxygen)
free radicals
infectious agents
physical and mechanical factors
nutrition imbalance
genetic factor
immunological reactions
often its from exposure to toxic chemicals, infections, or hypoxia
hypoxic injury can result from:
decreased amount of oxygen in the air
loss of hemoglobin
ischemia
decreased production of rbc (??? why is this here?? that’s not what ischemia is….)
inadequate blood supply to the area/tissue/organ
the most common cause of hypoxia
caused by arteriosclerosis and thrombosis
arteriosclerosis- gradual narrowing of arteries
thrombosis- complete blockage by blood clots
poisoning of oxidative enzymes (cytochromes)
diseases of CVS and/or respiratory systems
anoxia
total lack of oxygen
caused by total sudden obstruction (ex. embolus)
an acute obstruction in a coronary artery can cause myocardial infarction
cellular responses to hypoxia:
decreased oxygen leads to decrease in atp
low atp causes the plasma membranes sodium potassium pump and sodium calcium exchange to fail
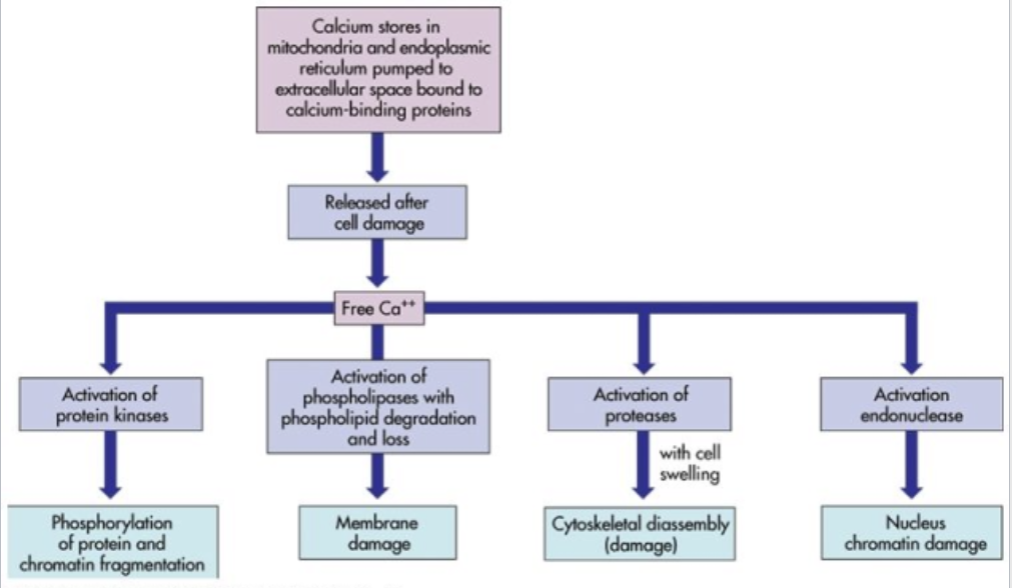
intracellular accumulation of Na+ and Ca2+
Na+ and water can enter the cell freely and lead to cellular swelling
reperfusion injury- injury caused by restoration of oxygen
membrane injury can be caused by free radicals such as reactive oxygen species
free radical- electrically uncharged atom or group of atoms having an unpaired electron
1 unpaired electron makes the molecule unstable so to stabilize itself, it either gives or takes an electron from another molecule (such as protein, lipid, or DNA)
this can disrupt a chemical bond and lead to:
lipid peroxidation- destruction of an unsaturated fatty acid
alteration of proteins (ex. fragmentation of polypeptide chain)
alteration of DNA (ex. breakage of single strands)
mechanisms for the inactivation of free radicals
some fatty acids of lipids contain double bonds
such bonds are venerable to be attacked by free radicals leading to lipid peroxidation
peroxide leads to membrane or organelle destruction
i have no clue what this has to do with a mechanism to inactivate a free radical…
body can sometimes rid itself of free radicals
chemical injury:
begins with biochemical interaction between a toxic substance and plasma membrane that leads to damage and increased permeability
can be caused by:
direct toxicity
reactive free radicals and lipid peroxidation
chemical agents that cause cellular injury:
lead
primarily hazardous to children, particularly to fetuses (the nervous system is vulnerable)
carbon monoxide
it interrupts respiration
ethanol (alcohol)
mercury
social or street drugs (ex. marijuana, cocaine)
unintentional or intentional injuries:
blunt force injuries are caused by mechanical force (ex. car accidents)
cause tearing, shearing, or crushing of tissues
the most common type of injury
some injuries caused by non sharp force:
contusion or hematoma- bleeding into skin or tissue
contusion = bruise
blood vessels were ruptured without breaking the skin
abrasion- removal of superficial layer of the skin
caused by friction between skin and the object that caused the injury
laceration- irregular tear or rip of skin or tissue
fracture- broken bone
contusions and hematoma:
bruising → extravasated rbcs (rbcs get outside where they’re supposed to be aka the blood vessel) → phagocytosis of rbcs by macrophages → (either/or) hemosiderin or iron-free pigments
the color changes are due to the hemoglobin changes and the breakdown of the extravasated blood (ex. the final hemoglobin breakdown product is bilirubin which is yellow so bruises are yellow/green in their last stages)
sharp force injuries:
incised wounds- a cut that is longer than it is deep
can be sharp or jagged
has sharp and distinct edges
stab wounds- a penetrating sharp force injury that is deeper than it is long
puncture wounds- a deep but relatively narrow penetrating wound
chopping wounds= combination of sharp and blunt force
gunshot wounds:
entrance wounds- caused by the bullet entering the body
contact range entrance wound
intermediate range entrance wound
tattooing and stippling
its not talking about wounds from tattooing and stippling, its a description of things around the wound site, ‘tattooing’ is when there is unburnt particles and metal scraps embedded in the surrounding skin (also maybe when there are small burns from burning gunpowder) (might also be stippling, unsure)
indeterminate range entrance wound
exit wounds- caused by the bullet exiting the body
shored exit wound- caused when the skin is contact with another object when the bullet exits; produced when the outstretched skin is impaled, sandwiched, and crushed between the outgoing bullet and an unyielding object over the exit site, thus leaving an abrasion collar on the wound margin
asphyxia injuries are caused by a failure of cells to receive or use oxygen
they are grouped into:
suffocation- can result from lack of oxygen in the air
strangulation- caused by compression and closure of blood vessels and air ways
chemical asphyxiants- prevent or block delivery of oxygen to the tissue (ex. carbon monoxide)
drowning
infectious injury
pathogenic (virulence) microorganisms:
invade and destroy cells
produce toxin
produce damaging hypersensitivity reactions
immunologic and inflammatory injury:
phagocytic cells cause injury to the cell
immune and inflammatory substances such as histamine, antibodies, lymphokines, complement, and enzymes can cause cellular injury
membrane alterations
injurious genetic factors:
nuclear alterations
alterations in the plasma membrane structure, shape, receptors, or transport mechanisms
aka genetic disorders
ex. sickle cell anemia, muscular dystrophy
injurious nutritional imbalances:
essential nutrients are required for cells to function normally
deficient intake (hypo-)
ex. hypolipidema
excessive intake (hyper-)
ex. hyperlipidemia
temperature extremes:
hypothermic injury- chilling or freezing of cells
slows cellular metabolic processes
hyperthermic injury- caused by excessive heat
heat cramps- cramps are a result of salt and water loss (ex. during vigorous exercise)
heat exhaustion- in addition to fluid loss, hypotension occurs
heatstroke- life threatening condition caused by high humidity and high temperature
atmospheric pressure changes:
sudden increases or decreases in atmospheric pressure
blast injury
decompression sickness or caisson disease (aka “the bends”)
ionizing radiation:
any form of radiation capable of removing orbital electrons from atoms
xrays, gamma rays, alpha and beta particles
DNA is the most vulnerable target
mechanism of damage
effects of ionizing radiation
manifestations of cellular injury:
cellular accumulations (infiltrations)- in addition to injury, cellular accumulations can occur as a result of normal cell function
common accumulations consist of:
water- cause (s?) cellular swelling
lipids and carbohydrates- as a result of some metabolic disorders
glycogen- as a result of genetic disorders
proteins
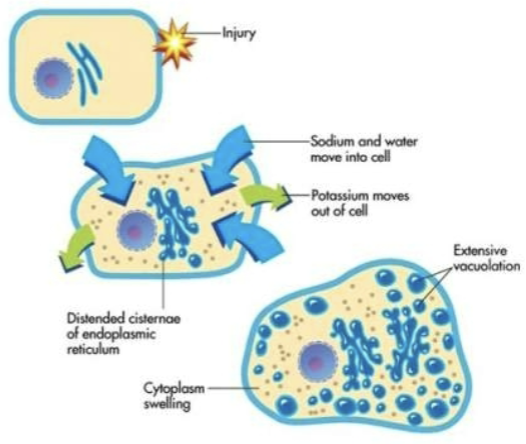
hydropic degeneration:
injury → hypoxia → atp production decreases → sodium and water move into the cell, potassium moves out of the cell → osmotic pressure increases → more water moves into the cell → cisternae of endoplasmic reticulum distend, rupture, and form vacuoles → extensive vacuolation → hydropic degeneration
the swelling from an influx of water that happens in an injured cell
cellular accumulations (infiltrations) (ig more??):
pigments
melanin, hemoproteins, bilirubin
calcium- accumulate in both injured and dead tissue
urate- hyperuricemia can cause gout (acute or chronic arthritis)
cellular death:
necrosis
sum of cellular changes after local cell death and the process of cellular autodigestion (aka self digestion)
irreversible injury progresses to necrosis
processes (i think all of these go under necrosis)
karyolysis
nuclear dissolution and chromatin lysis
pyknosis
clumping of the nucleus
condensation (clumping) of chromatin in the nucleus
karyorrhexis
fragmentation of the nucleus
necrosis:
the 4 major types of necrosis are:
coagulative necrosis- occurs primarily in kidney
protein denaturation
liquefactive necrosis- occurs in neurons and glial cells of the brain
hydrolytic enzymes
caseous necrosis
tuberculous pulmonary infection
combination of coagulative and liquefactive necrosis
fat necrosis
breast, pancreas, and other abdominal organs
action of lipases
apoptosis:
programmed cellular death
mechanisms (?)
necrosis vs apoptosis (?)
apoptotic signal → initiator caspase → death substrates → (either/or) disable DNA repair and cell survival proteins or condense chromosome and fragment DNA → (come back to later bc i’m confused, slide 48)
aging and altered cellular and tissue biology:
aging vs disease (?)
normal life span (?)
gender differences (?)
theories of aging:
accumulation of injurious events
genetically controlled program
theories
genetic and environmental lifestyle factors
alterations of cellular control mechanisms
degenerative extracellular changes
aging:
cellular aging
tissue and systemic aging
fraility
clinical fraility scale:
1- very fit
people who are robust, active, energetic, and motivated. these people commonly exercise regularly. they are among the fittest for their age
2- well
people who have no active disease symptoms but are less fit than category 1. often, they exercise or and very active occasionally (e.g. seasonally)
3- managing well
people whose medical problems are well controlled, but are not regularly active beyond routine walking
4- vulnerable
while not depending on others for daily help, often symptoms limit activities. a common complaint is being “slowed” up and/or being tired during the day
5- mildly frail
these people often have more evident slowing, and need help in high order IADLs (finances, transportation, heavy housework, medications). typically, mild fraility progressively impairs shopping and [finished from similar chart i found: walking outside alone, meal preparation, medications, and begins to restrict light housework]
6- (from a similar chart i found) moderate frailty
people who need help with all outside activities and with keeping house. inside, they often have problems with stairs and need help with bathing and might need minimal assistance (cuing, standby) with dressing
7- severely frail
completely dependent for personal care, from whatever cause (physical or cognitive). even so, they seem stable and not at a high risk of dying (within 6 months)
8- very severely frail
completely dependent, approaching the end of life. typically, they could not recover even from a minor illness
9- terminally ill
approaching the end of life. this category applies to people with a life expectancy of less than 6 months but are not otherwise evidently frail
scoring frailty in people with dementia:
the degree of frailty corresponds to the degree of dementia. common symptoms in mild dementia include forgetting the details of a recent event, though still remembering the event itself, repeating the same question/story and social withdrawal. [finished from a similar chart i found: in moderate dementia, recent memory is very impaired, even though they seemingly can remember their past life events well. they can do personal care with prompting. in severe dementia, they cannot do personal care without help. in very severe dementia, they are often bedfast (bedbound?) . many are virtually mute]