GCSE Chemistry Revision "Charges on Ions"
Introduction to Ionic Compounds
Ionic compounds are formed when metals react with non-metals.
Ions are charged atoms; metals form positive ions while non-metals form negative ions.
Metal Ions
Positive Charges
Examples of metal ions:
Sodium (Na⁺): Forms a +1 ion, located in group 1.
Magnesium (Mg²⁺): Forms a +2 ion, located in group 2.
Aluminium (Al³⁺): Forms a +3 ion, located in group 3.
Hence, the charge on a metal ion is often the same as the group number in the periodic table
Group Correlation
The charge on metal ions usually reflects the group number:
Group 1: +1 ions (e.g., Li⁺, Na⁺, K⁺)
Group 2: +2 ions (e.g., Be²⁺, Mg²⁺, Ca²⁺)
Group 3: +3 ions (e.g., Al³⁺, Ga³⁺)
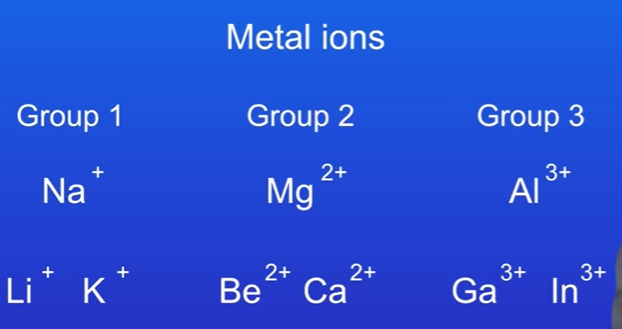
Magnesium is in group two and forms a 2 plus ion, and aluminium is in group three and that forms a 3 plus ion. We can also see this with other metals from these groups, which I'm showing you here. Both lithium and potassium are in group one and they form 1 plus ions. Beryllium and calcium are both in group two and they form 2 plus ions, and gallium and indium are in group three and these can form 3 plus ions.
Transition Metals
Unlike main group metals, transition metals can form several different ions:
Iron (Fe): Can form Fe²⁺ (iron(II)) and Fe³⁺ (iron(III)).
Copper (Cu): Can form Cu⁺ (copper(I)) and Cu²⁺ (copper(II)).
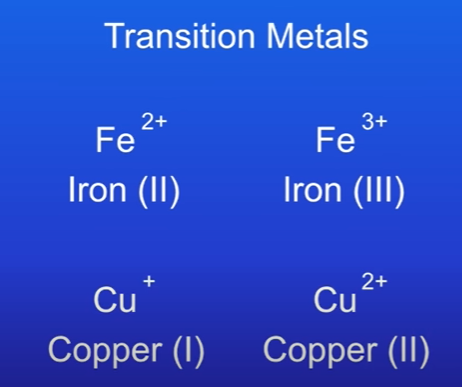
iron; this can form a 2 plus ion and a 3 plus ion. Scientists call these iron(II) and iron(III)
copper, which can form a 1 plus ion and a 2 plus ion, and again we call these copper(I) and copper(II)
Non-Metal Ions
Negative Charges
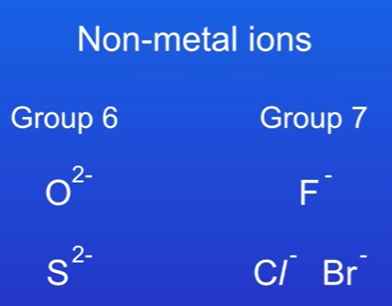
Examples of non-metal ions:
Oxide ion (O²⁻): Group 6, forms a -2 ion.
Fluoride ion (F⁻): Group 7, forms a -1 ion.
Group Correlation
Non-metal ions charge reflects the group number:
Group 6: Forms -2 ions (oxygen, sulfur as sulfide S²⁻).
Group 7: Forms -1 ions (fluorine, chlorine, bromine).
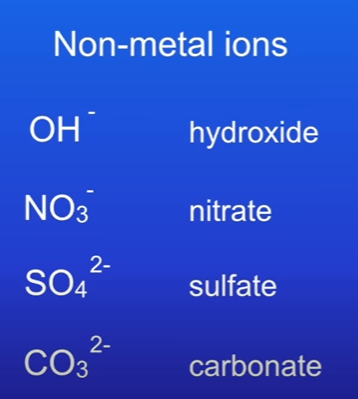
Complex Non-Metal Ions
there are some non-metal ions which consist of several non-metal atoms chemically combined, here:
There are a couple of non-metal ions which are different from the rest, because they're positive, thus they are positively charged non-metal ions, unlike the rest of the non-metal ions which are negative, these ions are: These ions are the hydrogen ion H⁺ and the ammonium ion NH₄⁺

Exceptions: Positively charged non-metal ions:
Hydrogen ion (H⁺)
Ammonium ion (NH₄⁺)
Conclusion
Recap: Metal ions are positive and most non-metal ions are negative, correlating with their group numbers in the periodic table.
Upcoming content will focus on calculating the formulas of ionic compounds, such as sodium sulfate (Na₂SO₄) and calcium hydroxide (Ca(OH)₂).