topic 2 : notes
PART A
chemical reactions are always occurring in the human body
mass: a measure of the amount of material in an object
3 forms : solid, liquid, gas
element: a substance that cannot be broken down into other substances by chemical reactions; only of atoms with the same number of protons.
ex> all atoms with six protons in their nucleus are carbon atoms, no matter how many electrons or neutrons they have
all matter is composed of chemical elements
atomic number of an element: the number of protons in the nucleus of an atom of that element
number on top
= p only
mass number of an element: the total number of protons and neutrons present in the nucleus of an atom of that element
number on bottom
= p + n
4 most abundant / important elements make up about 96% of the weight of the body
oxygen (O)
carbon (C)
hydrogen (H)
nitrogen (N)
elements combine to form compounds
ex> H2O = water
atom is the smallest unit of matter
atoms are composed of 3 subatomic particles:
proton: (+) positively charged
determine element
electron: (-) negatively charged
participate in chemical reactions
outer-shell electrons determine chemical behavior
neutron: neutral / no charge
determine isotope
when an atom has an equal number of protons and electrons,
its net electrical charge is ZERO and its NEUTRAL
nucleus: is the atom’s central core ; consists of neutrons and protons
electrons move around the nucleus
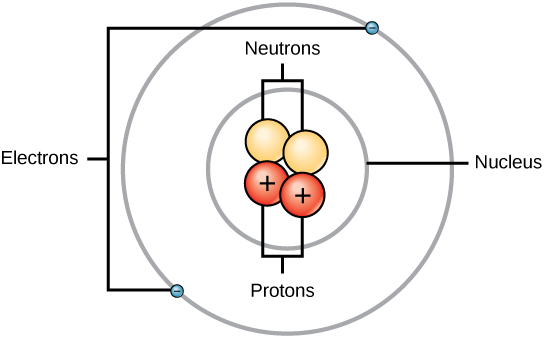
usually the number of neutrons is equal to the number of electrons, EXEPT for an isotope
isotopes: atoms of the same element that have different numbers of neutrons
ex> one carbon atom may have six neutrons, and another may have seven
only electrons are directly involved in chemical reactions
they determine the chemical properties of an atom
enable atoms to transfer or share electrons
these interactions result in atoms staying together and are held by attractions called chemical bonds
3 types of chemical bonds
ionic bonds
when an atom loses or gains electrons, it becomes electrically charged or POLAR
ionic bonds are atoms or molecules that are electrically charged as a result of gaining or losing an electron
compounds, ex table salt, that are held together by ionic bonds are called ionic compounds

covalent bonds
when two atoms share one or more pairs of electrons
POLAR covalent bonds: electrons are shared unequally between atoms due to a difference in electronegativity
NONPOLAR covalent bonds: electrons are shared equally between atoms with similar electronegativity
the strongest of bonds
hold atoms together in a molecule
electrons are the dots that are shared
ex> formaldehyde (CH2O)

hydrogen bonds
Attraction between a covalently bonded hydrogen atom and another atom taking part in a separate covalent bond.
polar molecules; with uneven distribution of chargepolar has both negative and positive charge( + & - )
ex> water
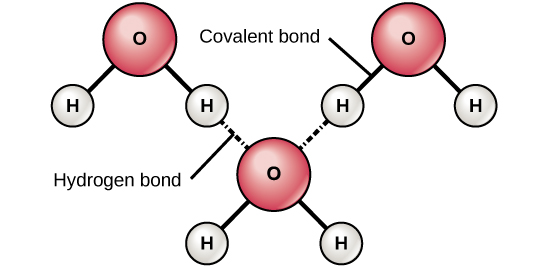
the polarity of water results in weak electrical attractions between neighboring water molecules
these weak attractions are the hydrogen bonds
Chemical Reactions
Chemical Reactions: cells constantly rearrange molecules by breaking existing chemical bonds and forming new ones
include
reactants: the starting material (string or beads)
products: the end material (a necklace)
can rearrange matter
CANNOT CREATE OR DESTROY matter
Why is water so important?
the polarity of water molecules and the hydrogen bonding that results explain most of water’s 4 life-supporting properties
the cohesive nature of water
cohesion: the tendency of molecules of the same kind to stick together
water is much stronger than most other liquids
a drop of water on a glass slide will stay a drop of water; a drop of alcohol will run onto the sides of the glass
trees depend on cohesion to help transport water to their roots and leaves
the hydrogen bonding makes it stick together
surface tension: a measure of how difficult it is to stretch or break the surface of a liquid
hydrogen bonds give water unusually high surface tension and make water behave as though it were coated w invisible film

the ability of water to moderate temperature
because of hydrogen bonding, water has a stronger resistance to temperature change
it takes extended periods of time for the temperature of water to change (ex> a pond when it rains)
means water has a high heat capacity
when water is heated, the heat energy:
disrupts hydrogen bonds
then makes water molecules jostle around faster
water absorbs and stores a large amount to heat while warming up only a few degrees
ex> some electric cars run cool water around when over heated because the water absorbs the heat
evaporated cooling: when a substance evaporates and the surface of the liquid remaining behind cools down
ex> sweating
the biological significance of ice floating
solids usually sink, but ice is less dense than liquid water
water molecules are FURTHER away and move SLOWER
this property of water allows organisms to survive, ex sea lions, in extremely cold temperature seasons or areas

when a deep body of water cools and a layer of ice forms on top, the floating ice acts as an insulating “blanket” over the liquid water, allowing life under the frozen surface
if ice did not float, oceans, lakes, and ponds would freeze solid and kill the organisms
the versatility of water as a (universal) solvent
solution: a liquid consisting of a homogenous mixture of 2 or more substances
solvent: the dissolving agent
solute: the dissolved substance
ex> water is a solvent used to dissolve sugar, the solute
the solution is sugar water
OIL DOES NOT DISSOLVE IN WATER, FATS DO NOT DISSOLVE IN WATER
ex> salt (NaCl) water
mickey mouse ears, Hydrogen+, surrounds Cl- charge
mickey mouse face, Oxygen- , surrounds Na+ charge
Acids, Bases, & pH
acid: chemical compound that releases H+ to a solution
more H+
base: a compound that accepts H+ and removes them from a solution
more OH-
pH scale: measures the acidity of a solution
scale ranges from 0 (most ACIDIC) to 14 (most BASIC)
each pH unit represents a tenfold change in the concentration of H+
ex> lets say seawater has a pH 8 & urine has a pH 6,
seawater is 100x more basic than urine (10×10)
how much more basic is pH14 oven cleaner than pH11 milk of magnesia?
1,000 fold (10×10×10)
take the number (14-11 =3) & multiply 10 that many times
pure water is pH 7 , neutral
equal

battery acid is pH 1 | stomach acid is pH 2 | human blood is pH 7.4 | urine pH 6.8
PART B
lactose: the main sugar found in milk
lactose intolerance: the inability to properly digest lactose
most people are as adults
a cell is mostly water
the rest of the cell consists of mainly carbon-m based molecules
carbon forms large, complex, & diverse molecules necessary for life’s functions
organic compounds: carbon- based molecules
carbon is a versatile molecule
carbon can share electrons w other atoms in four covalent bonds (double bonds)
because carbon can use one or more of its bonds to attach to other carbon atoms, it is possible to construct an endless diversity of carbon skeletons varying in :
size
branch pattern


carbon atoms of organic compounds can also bond w other elements, most commonly
hydrogen
oxygen
nitrogen
on a molecular scale, many of life’s molecules are gigantic, earning the name macromolecules
polymer: made by stringing together many smaller molecules called monomers (poly: many)
monomers: subunit of a polymer (mono: one)
dehydration reaction:
links 2 monomers together (2 beads)
removes a molecule of water
organisms also have to break down macromolecules (removing the beads)
Hydrolysis: BREAKS bonds between monomers of a polymer,
adds a molecule of water
reverses the dehydration reaction
digestion breaks down macromolecules to make monomers available to your cells
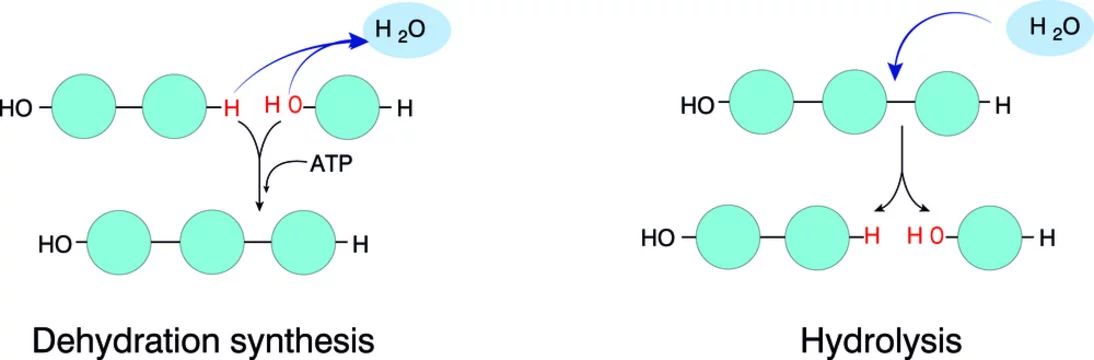
4 categories of macromolecules:
carbohydrates : sugars and polymers of sugar
primary source of dietary energy
ex> small sugar molecules in soft drinks
long starch molecules in pasta and bread
almost all carbs are hydrophilic (water loving) that dissolve in water easily
monosaccharides: (one) the SIMPLEST carb molecule (individual bead)
particularly glucose is the universal food; main fuels for cellular work
fructose is found is fruit
disaccharide: DOUBLE sugar from two monosaccharides by dehydration reaction
sucrose: when glucose and fructose are combined,
(ex> table sugar, cane sugar, beat sugar, & can be found in honey)
main carb in plant sap
table sugar is 50% fructose 50% glucose
HFCS is 55% fructose & 45% glucose
hence the name high fructose
lactose: combination of galactose and glucose
(ex> milk sugar)
maltose: 2 glucose linked together
(ex> beer, malted milk shakes)
polysaccharides: 3 or more (complex) monosaccharide linked chains
starch: long string of glucose monomers
potatoes & grains
glycogen: “animal starch”
used by animals to store energy
glycose is broken down to release glucose when you need energy
cellulose: the most abundant organic compound on earth (ex> grass, wood)
forms cable like fibrils in the walls that enclose plant cells
cannot be broken by any enzyme produces by animals
only cows and termites can break it down because they have special bacteria in their digestive systems
lipids (fats) : hydrophobic (water resisting) unable to mix with water
oil floats on top of vinegar in dressing
lipids differ from from the other macromolecules because they are neither huge nor are necessarily polymers built from repeating monomers
some lipids are not assembled as polymers
lipids are a diverse group of molecules made from different molecule building blocks
a typical fat, triglyceride (tri=3), consists of glycerol molecule joined with three fatty acid molecules via dehydration reaction
saturated fats: do not have a double bond
maximum number of hydrogens
ex> butter, lard; solid at room temp
animal fats
unsaturated fats: have a double bond
has fewer than the maximum number of hydrogens
ex> olive oil, peanut oil ; liquid at room temp
most plant and fish fats
steroids: carbon skeleton has 4 fused rings
cholesterol: key component of cell membrane
estrogen & testosterone are technically fats
synthetic anabolic steroids
variant of testosterone that mimics the effects
abused by athletes to build muscle quickly
functions of fat that are essential in the human body
energy storage
contains more potential energy than carbohydrates
cushioning
insulation
proteins : polymers of amino acid monomers
account for 50% of the dry weight of most cells
instrumental in almost everything you do
5 functions of proteins:
structural proteins > provide support
storage proteins > provide amino acids for growth
contractile proteins > helps movement
transport proteins > help transport substances
enzymes > help chemical reactions
all proteins are made by stringing together 20 kinds of amino acids (necklace w 20 diff colors)
their order determines the 3D structure of the protein
the proteins 3D structure enables the molecule to carry out its specific function
nearly all proteins work by recognizing and binding to some other molecule
a change in the sequence can affect a protein’s ability to function
ex> sickle-cell anemia
every amino acid consists of a central carbon atom bonded to 4 covalent partners
for humans: there are 9 essential amino acids that you must get from diet; your body can synthesize the other 11 amino acids as needed
three attachment groups for amino acids:
carboxyl group (-COOH)
amino group (-NH2)
hydrogen atom
cells link amino acids together by dehydration reactions
forming peptide bonds
creating long chains of amino acids called polypeptides
nucleic acids : macromolecules that
store information
provide instructions to build proteins
two types
DNA > deoxyribonucleic acid; genetic material inherited from parents
gene: discrete unit of inheritance encoded in a specific stretch of DNA that programs the amino acid sequence of a polypeptide
those programmed instructions are written in a chemical code that must be translated
from “nucleic acid language ” to “protein language”
each DNA nucleotide has 1 of 4 possible nitrogenous bases
adenine (A)
guanine (G)
thymine (T)
cytosine (C)
A always pairs with T
G always pairs with C
form covalent bonds between the sugar of one nucleotide and the phosphate of the next
form a sugar-phosphate backbone ; bases (A,T,C or G) hang off the backbone like appendages
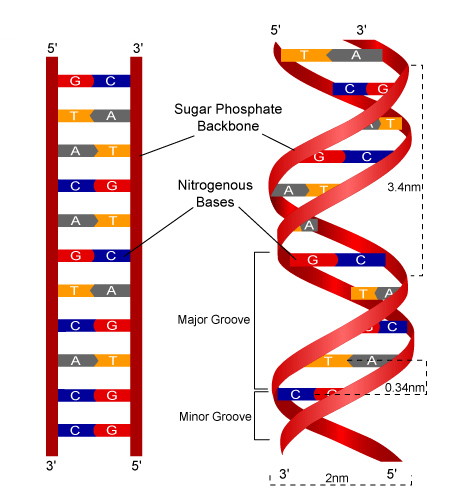
RNA > ribonucleic acid ; single stranded
nucleic acids are polymers made from monomers called nucleotides
each nucleotide has 3 parts
five-carbon sugar
phosphate group
nitrogen-containing base