GEOL 101 Exam 1 Review
Chapter 1: The Universe and Earth
Big Bang (13, 15)
The Big Bang Theory proposes that all matter and energy in the Universe started out as a single infinitesimally small point.
The galaxy continues to expand.
First element formed = Hydrogen
Hydrogen fused to helium, initiating a star.
Stars, supernovas, and the formation of elements (18, 19)
Stars have a finite amount of hydrogen.
Hydrogen is their main fuel.
When it runs out of hydrogen, it gets dark.
When supermassive stars die, they explode in a supernova.
Big Bang nucleosynthesis formed the lightest elements.
Hydrogen and Helium.
Stella nucleosynthesis led to fusion of elements during the life cycle of a star.
Up to Iron (Fe)
Elements with atomic numbers larger than 26 formed during supernovae nucleosynthesis (atomic number 27 to 92).
Nebular theory - formation of a solar system (22)
Cosmic dust - a mass of hydrogen, helium, and other elements.
Cosmic dust and gas begin clinging together due to electrical charges that act on them.
They build a mass and collect more debris, forming a planet.
When that happens, the mass causes the hole of the nebulous cloud to start rotating around and flatten out.
Mass of sun, composition of planets – refractory vs. volatile (24)
99.98% of all of the material that was once in the nebulous cloud is now held within the sun.
A blast sent out lighter elements and pushed them to farther parts of the solar system.
The heavier elements, refractory elements, came together to make up rocky bodies.
Four terrestrial planets (Mercury, Venus, Earth, Mars) are smaller than the gas planets.
All condensed down under refractory materials.
Differentiation (28)
When our planet began forming, denser material started to sink to our core.
Heat caused the material to flow under gravity.
Differentiation is the organization of the Earth into layers.
Led to the formation of a core, a crust, and eventually continents.
The light elements were driven from the interior to form an ocean and atmosphere.
Denser elements = core
Lighter elements = surface
Differentiation created the magnetosphere, atmosphere, and our tectonic plates.
We are the stars (30)
Dust particles and stony debris from supernovae coalesced to create the planetsimals that amassed together to form the Earth.
Our planet and ourselves are made up of the elements of exploded stars.
Asthenosphere, lithosphere, and Earth’s layers (49, 50)
Using sound waves, we can know what the interior of the Earth looks like.
The interior is divided up in layers:
Crust - made out of lighter minerals
Upper mantle
Transition zone
Lower mantle
Outer core - liquid
Inner core - solid
By volume, the most of our planet exists in the mantle.
Lithosphere - the crust and upper mantle, both act rigid.
Asthenosphere - the soft layer in the lower part of the mantle.
Where melting occurs.
Our tectonic plates are comprised of the lithosphere.
Below the tectonic plates is the asthenosphere.
Tectonic plates are moving and recycling material and hydrating out the lighter materials.
Continental crust - thicker, less dense.
Oceanic crust - thinner, denser.
Compare compositions and density to Earth’s layers, and geothermal gradient (51)
The mantle comprises most of the Earth’s mass.
The outer core is liquid.
The inner core is solid.
The temperature and the pressure both increase as we go deeper within the Earth, toward its core.
Chapter 3: Minerals
Definition of a mineral – 5 criteria (4)
Naturally occurring
Formed by geologic processes
Inorganic
Crystalline solid
Definite chemical composition
What qualifies as a mineral (10)
Table salt
Crystal formation (12)
Minerals are composed of 1 or more elements, the atoms are bound together by chemical bonds.
A crystal is a single continuous piece of crystalline solid typically bounded by flat crystal faces.
Minerals are not always found as perfect, full crystals.
Crystal faces grow naturally as the mineral forms and reflect atomic structure, growing in an orderly arrangement of atoms.
Example: prismatic
Its touching silicon and oxygen ions in a scaffolding way.
The geometry of the atomic arrangement defines the crystal structure and the nature of chemical bonding determine the mineral properties.
Lattice structures give us sets of properties for us to test minerals (clues on how to identify mineral).
Atomic bonding, polymorphs of Carbon (13)
Covalent bonds in a mineral can determine strong the mineral is.
Molecules help form a pattern in the structure of a mineral.
The two polymorphs of carbon (diamond and graphite) are the hardest and softest minerals, a result of different types of chemical bonds.
Color is not a good way to identify minerals, do not look at color first. Many minerals vary is color. Quartz can be any color, for example.
Different minerals grow different crystal shapes.
Crystal habit - is the ideal shape of the crystal.
Euhedral vs. anhedral crystals (19)
Euhedral crystals - Minerals with well-formed crystal faces.
Anhedral crystals - Minerals without well-formed crystal faces.
Different settings where minerals form (20-24)
Solidification - occurs when molten rock, such as lava or magma, cools and different minerals grow in succession.
Can happen below the earth - intrusive.
Can happen above the Earth’s surface - extrusive.
Certain minerals crystallize at certain temperatures as the magma is cooling down.
Precipitation - occurs from volcanic gas, deep sea, hydrothermal vents, or element-rich gas. Occurs when water in a salty desert undergoes evaporation.
Bio-mineralization - Refers to the production of minerals by organisms. Reef organisms, extra ions from water to make shells.
Diffusion - Metamorphic process, atoms migrate through the crystal and new minerals grow inside the rock, happens slowly.
3 ways in which minerals can be destroyed:
Water
Erosion
Heat/melting
Physical properties - represent crystal structure and chemical composition (28-37)
What to look at when identifying minerals:
Color - a diagnostic of some minerals (turquoise) but a poor indicator for others (quartz).
Hardness - The scratching resistance of a mineral, which is directly linked to chemical bond strength.
Streak - The color of a mineral when it is powered (rubbing it on an unglazed porcelain plate).
Luster - Luster refers to the way that a mineral surface scatters light.
Cleavage - The tendency for a mineral to breakalong lattice planes with weaker atomic bonds.
Fracture tendency - Minerals fracture when they break through the lattice planes instead of along them.
Reaction to acid
Crystal habit
Silicate minerals are the most common rock forming mineral group on Earth (45-48)
Silicate minerals are by far the most dominant substances comprising Earth’s crust (90%) and mantle (>99%).
Silicon and oxygen account for more than 74% of crustal mineral mass.
Silica tetrahedron
Isolated tetrahedron
Single chain
Double chain
Sheet
Framework
Minerals are important! (60)
Minerals make up many items we use on a daily basis (phones, counters, wires, construction equipment).
Chapter 4: Igneous Rocks
Cemented vs. crystalline rocks (4, 5)
Crystalline rocks are held together by interlocking crystals.
Cement - like glue, it is deposited between little tiny pore spaces between crystals.
Intrusive vs. extrusive, and connect that to igneous rock texture (15)
Lava is found on Earth’s surface.
Magma is found below the surface.
The extrusive realm is above ground.
The intrusive realm is below ground.
Results in crystals
How melting occurs – decompression, flux, heat transfer – and the settings where these occur (22-26, 28)
Decompression melting
Pressures decreases but temperature remains constant, creating a melt.
Changing pressure (relieving pressure), not temperature.
Where: Geologic environments where decompression melting occurs:
Decompression melting occurs at mantle plumes, continental rifts, and divergent-plate boundaries.
Rift zone - where Earth is being pulled a part.
Decompression melting happens at mid-ocean ridges.
Black rocks from decompression melting spill on surface (basalt).
Add volatiles (flux)
Volatiles help break chemical bonds, creating a melt (molten rock).
Volatiles are gaseous components of magma that can vaporize at surface pressures (can easily vaporize).
Takes place at subduction zone.
Volatiles such as H2O and CO2 are driven from the oceanic crust into the asthenosphere, creating a melt above the sub-ducting plate.
Flux happens along subduction zones
Heat transfer (conduction)
Heat from magma melts adjacent rocks, more melting.
Rocks surrounding magma chambers can be melted through heat-transfer.
Sharing heat.
Silica content composition (mafic → felsic) (32, 34)
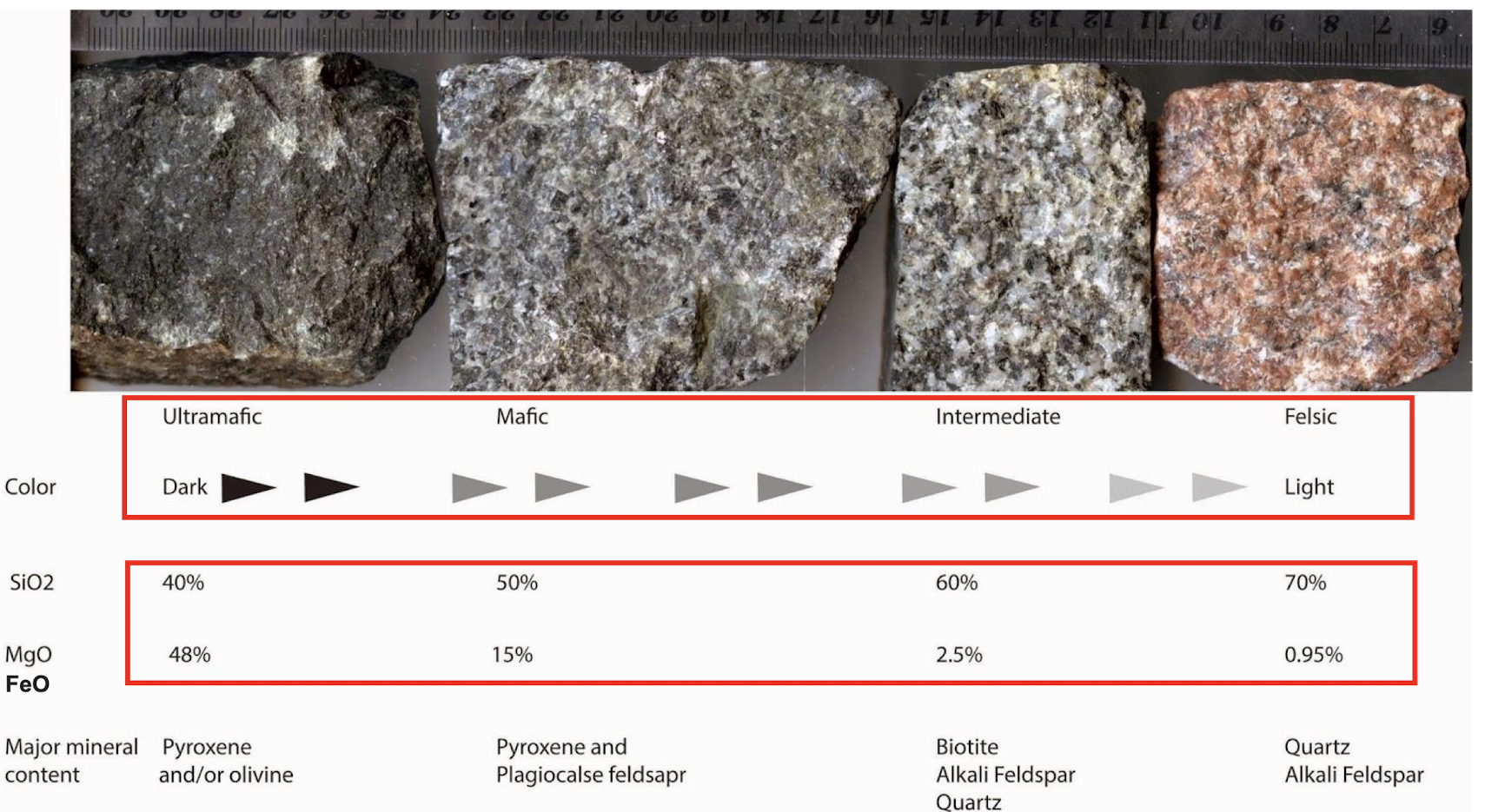
Partial melting and resulting composition (36)
Based on base rock/source rock.
The source rock dictates the initial melt composition.
Partial melting of rocks makes the melt silica-enriched because felsic minerals melt first.
Low temperature rocks.
Partial melting - when heat is introduced to a solid rock (source rock).
Viscosity of different types of lavas (42, 43)
The resistance to flow, or viscosity of a liquid affects the speed at which the liquid moves.
Viscous - having a thick, sticky consistency between solid and liquid; having a high viscosity.
Not all molten rock has the same viscosity. The viscosity of molten rock depends primarily on its:
Temperature - a lower temp melt is more viscous than a higher temp melt.
Volatile content - a wet melt containing more volatiles is more viscous than a dry (volatile free) melt.
Silica content - a felsic melt is more viscous than a mafic melt, because relatively more silicon-oxygen tetrahedra occur in a felsic melt.
Bowen’s reaction series (49)
Rocks formed from the top of the series are felsic.
Rocks formed from minerals at the bottom of the series are mafic.
From top to bottom (low to high temperature):
Felsic (high silica content)
Intermediate
Mafic
Ultramafic (low silica content)
Intrusive igneous bodies (dikes, sills, plutons) (58, 59)
Dikes run vertically and cut across rock layers.
Sills run horizontally and are parallel to rock layers.
Dikes can spread rocks apart sideways, sills push rocks up and can change the relief.
Plutons are blob-shaped intrusions that solidify from magma chambers.
Igneous rock texture (phaneritic, aphanitic) (67-69)
Aphanitic - extrusive, formed outside, fine-grained
Phaneritic - intrusive, formed inside, coarse-grained
Chapter 5: Volcanoes
Olympus mons on Mars (6)
Largest volcano in our solar system.
Types of volcanoes (shield, cinder cone, stratovolcano, supervolcano, hot spot, mid-ocean ridge) and examples of each (8-13)
Shield
Broad, gentle domes whose shape resembles a soldier's shield lying on the ground.
Form when the products of eruption have low viscosity and can't build into a mound at the vent.
Constructed by lateral flow of low viscosity, basaltic lava (mafic)
Flow easily and spread out in thin sheets over large areas
Example: Kilauea (Hawaii)
Cinder cone
Cone-shaped piles of ejected basaltic lapilli-sized fragments that have built up at the angle of repose around the vent.
Steep slopes, maximum slope that the loose fragments can sustain before sliding down, smallest type of volcano.
Stratovolcano
Composite volcanoes
Constructed from alternating layers of high viscosity, andesitic or rhyolitic lava (felsic), tephra, ash, and debris.
Largest, most explosive, viscous, steep slopes
Example: Mt. St. Helens, Mt. Fuji, Mt. Rainier, Mt. Vesuvius
Supervolcano
Hot spot
Oceanic hot spot volcano forms on oceanic lithosphere, basaltic magma erupts at the surface on the seafloor.
Continental hot spots and rifts produce both effusive and explosive eruptions.
Mid-ocean ridge
Develop along fissures parallel to the ridge axis.
Erupt basalt which cools quickly underwater and forms pillow lava mounds.
Water heats up as it circulates through the crust near the magma chamber bursts out of hydrothermal vents along the mounds.
Calderas (19)
An enormous volcanic depression, much larger than a crater.
Forms when a magma chamber empties and the volcano collapses into the evacuated space.
A large circular depression with steep walls and a fairly flat floor, formed after an eruption as the center of the volcano collapses into the drained magma chamber below.
Example: Crater lake, Oregon and Yellowstone National Park.
Viscosity of magma/lava, effusive vs. explosive (23)
Effusive eruption
Low viscosity lava spills or fountains steadily from a vent or fissure.
Mafic.
Shield, fissures, mid-ocean ridges.
Basaltic lava flows, low silica, moves fast.
Explosive eruption
Pyroclastic debris blasts forcefully into the air.
Andesitic lava flows, viscous, not fast, mounds around vent.
Rhyolitic lava flows, most viscous, slowest (rarely flows), lava plugs vent as lava dome.
Explosions due to pressure.
Types of effusive basaltic lava (pahoehoe, A’a’) (25, 26)
Pahoehoe - basalt lava with ropy texture. Forms when extremely hot basalt cools, rolled ridges and furrows result in the cooling process because the lava is still flowing.
A’a’ - Basalt lava that solidifies with a jagged, sharp, angular texture. Forms from Pahoehoe.
Columnar jointing (28)
Columnar jointing - a type of fracturing that yields roughly hexagonal columns of basalt; columnar joints form when a dike, sill, or lava flow cools.
Pillow basalt (29)
Pillow basalt - when lava cools quickly in water, submarine basaltic lava travels short distances before freezing, which produces a glass-encrusted blob. Glass rind of pillow momentarily stops the flow, then pressure from the lava squeezing into the pillow breaks the rind and a new blob squirts out. Process repeats.
31 – Explosive volcanic textures (pyroclastic, tuff, obsidian – volcanic glass)
Pyroclastic - mix of rock fragments, pumice, and volcanic ash.
Tuff - volcanic ash and fragmented pumice, when debris accumulates and cements together, glass shards.
Tephra - volcanic deposits of pyroclastic debris of any size.
Obsidian - volcanic glass.
32 – Explosive volcanic features - lahar, debris flow, ash fall, pyroclastic flow
Lahar - A muddy, rapidly flowing slurry caused by ash-rich debris becoming very wet.
Debris flow - wetted debris that moves downhill. Moves like wet concrete.
Pyroclastic flow - an avalanche of hot ash, gas, and debris. Pompeii.
38-43 – Volcanism at tectonic plate boundaries & where mafic, felsic, intermediate rocks occur (basalt, andesite, rhyolite)
Mid-ocean ridges
MOR-generated oceanic crust covers 70% of earth - largest magmatic systems from decompression melting of mantle rock.
Low silica
Low heat
Mafic lava (fast)
Low volatiles
Low viscosity
Effusive
Basalt rocks
Convergent boundaries (arc)
Most stratovolcanoes form at convergent boundaries, from flux melting in the lithosphere.
High silica
Low heat
Felsic lava (slow)
High volatiles
High viscosity
Explosive
Andesite and Rhyolite
Continental rift zones
Caused by fractional crystallization and assimilation or heat transfer
melting continental crust
High silica
High and low heat (when it sits in a chamber to cool longer)
Felsic lava (slow)
Low volatiles
Low viscosity
Effusive
Basalt
Hot spots
Decompression melting in asthenosphere and lithosphere creates large volumes of magma
High and low heat
High volatiles
High viscosity
Explosive
Basalt
44, 45 - Famous volcanoes – Hawaii, Iceland, Mount St. Helens, Yellowstone
Iceland - mantle plume hot spot coinciding with a mid-ocean ridge.
Yellowstone - Mantle plumes that cut through continental crust create large volumes of felsic magma.