Human Physio Renal function and fluid balance
Structure & Function of the Urinary System
Functions
- Regulation of extracellular fluid volume & blood pressure
- works with CVS to ensure tissues get enough O2 and BP within normal values
- Regulation of plasma osmolarity
- Maintenance of ion balance
- in response to diet intake, urinary loss helps to maintain proper levels of Na+, K+, Ca2+ ions
- Homeostatic regulation of pH
- remove or conserve either H+ or HCO3- (bicarbonate ions) as needed
- Excretion of waste
- removes metabolic wastes dissolved in plasma e.g. uric acid and creatine
- Secretion of hormones and enzymes
- erythropoietin (RBC production), renin (sodium balance and BP homeostasis) & vit D conversion to control Ca2+ balance
Structure of nephron
the nephron is the functional unit of the kidney
Renal corpuscle filters blood plasma:
- Glomerulus capillary network
- Glomerular (Bowman’s) capsule double walled up surrounding glomerulus
Renal tubule filtered fluid passes into:
- proximal convoluted tubule
- descending, loop of henle (nephron loop) and ascending
- Distal convoluted tubule
Distal convoluted tubule of several nephrons empty into single collecting duct
Renal exchange processes
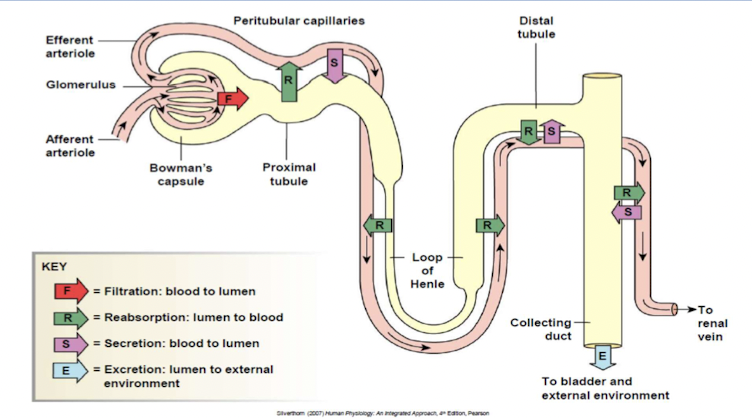
Urinary excretion of substance depends on its filtration, reabsorption and secretion.
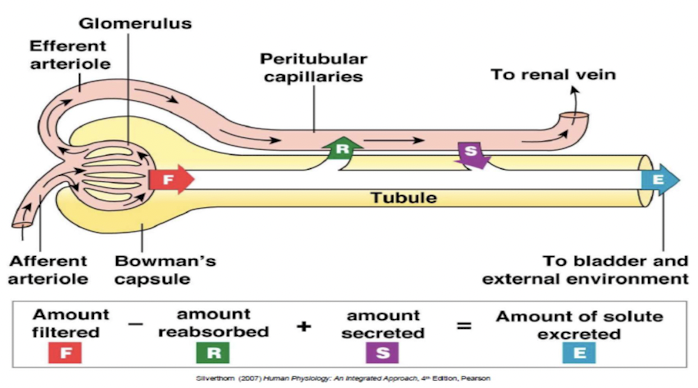
Glomerular filtration
Filtration barriers:
Glomerular capillary endothelium
Basal lamina
Epithelium of Bowman’s capsule- podocytes
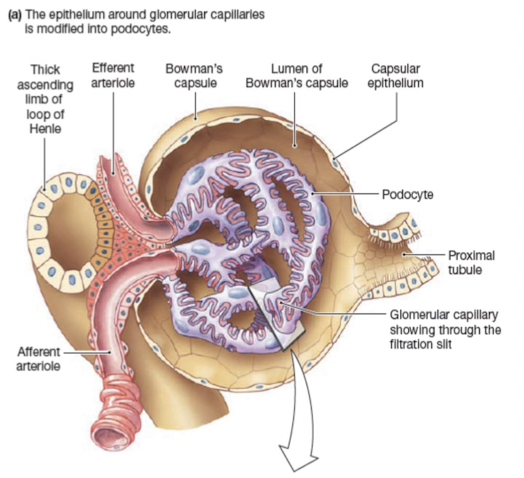
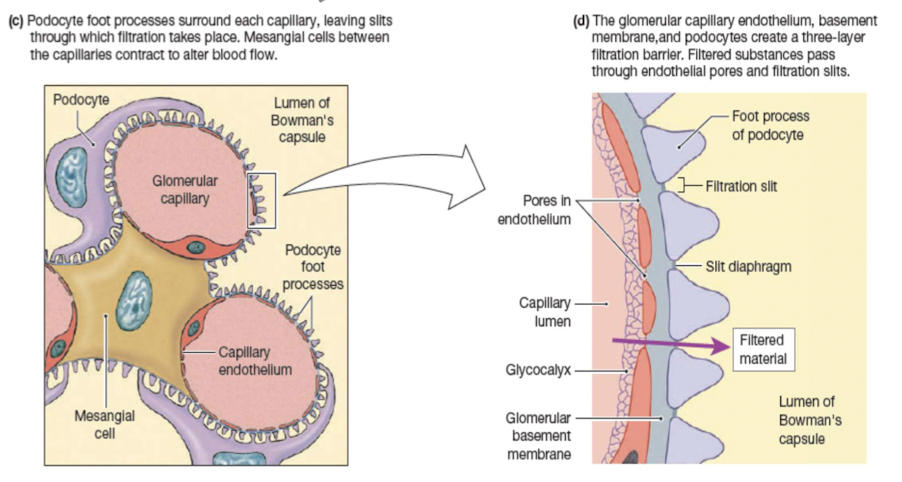
Forces that influence glomerular filtration:
- Hydrostatic/blood pressure (55 mmHg)
- pressure of flowing blood in glomerular capillaries
- favors movement of filtrate into Bowman’s capsule
- Colloid osmotic pressure (30 mmHg)
- plasma proteins entering capsule create a gradient that favors movement back into capillaries
- Hydrostatic fluid pressure (15 mmHg)
- in fluid up in enclosed Bowman’s capsule
- creates a gradient that favors movement back into capillaries
Glomerular filtration rate (GFR)
volume of fluid that filters into Bowman’s capsule per unit time
average GFP 125mL/min or 180L/day
Factors influencing GFP
- Net filtration pressure
- Filtration coefficient
- surface area of glomerular capillaries
- permeability between capillary & Bowman’s capsule
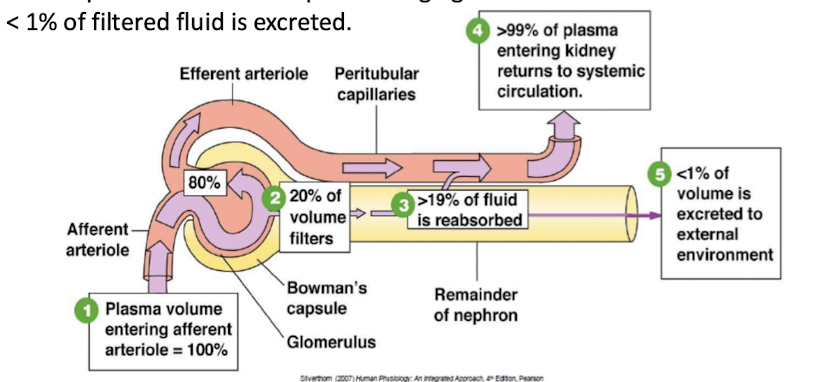
20% of plasma volume that pass through glomerulus is filtered
<1% of filtered fluid is excreted
Autoregulation of glomerular filtration rate takes place over a wide range of blood pressures.
Regulation of glomerular filtration rate
Myogenic response
- Intrinsic ability of vascular smooth muscle to respond to pressure changes
- ↑afferent arteriole resistance →↓GFR
- ↑efferent arteriole resistance → ↑GFR
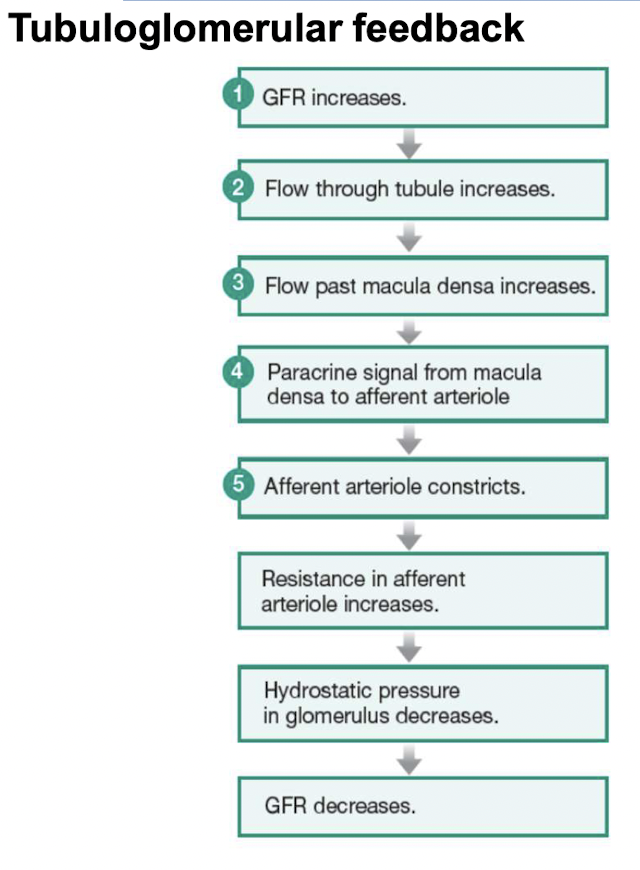
Tubuloglomerular feedback
Paracrine control through loop of Henle
Hormones and autonomic neurons
- By changing resistance in arterioles
- By altering the filtration coefficient
- ==Angiotensin II== - vasoconstrictor
- ==Prostaglandins== - vasodilators
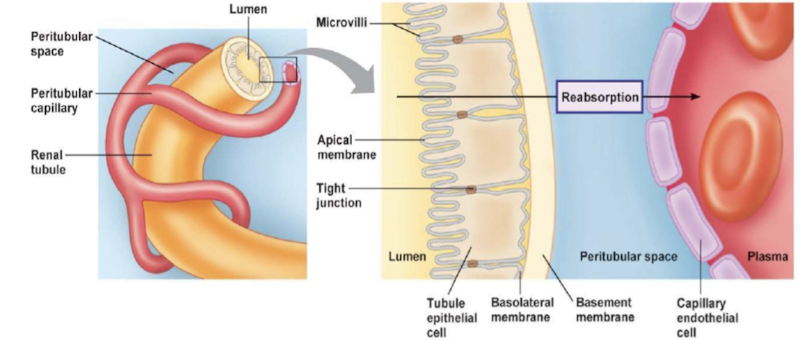
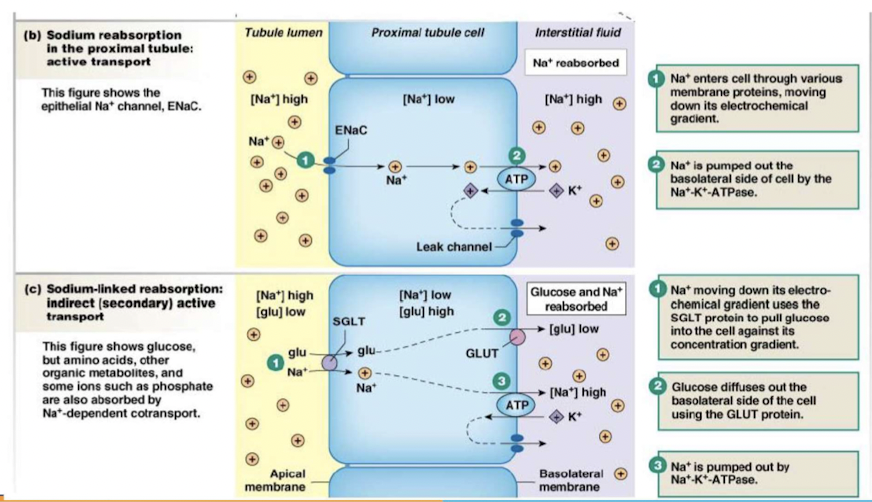
Reabsorption
movement of filtered solutes and water from lumen of tubule back into plasma
takes places in proximal tubule and distal segment of nephrons
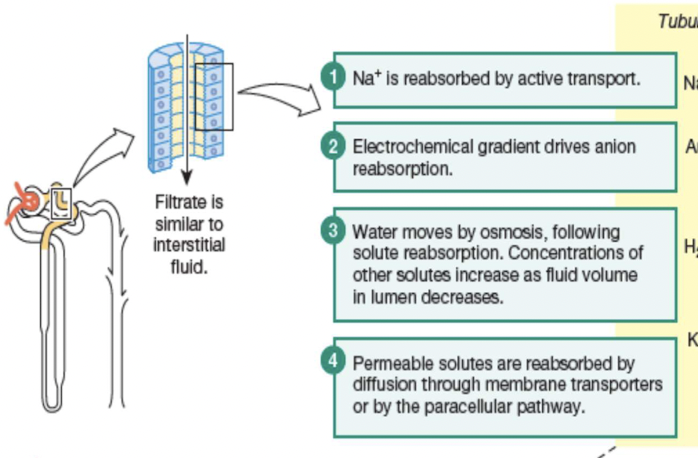
Principles governing tubular reabsorption of solutes & water:
- active transport to create concentration of electrochemical gradient
- water osmotically follow solutes
- Transepithelial transport (passing through cells)
- substances cross both apical and basolateral membrane
- Paracellular pathway (passing around cells)
- substances pass through junction between two adjacent cells
Principles governing the tubular reabsorption of solutes:
Some solutes and water move into and then out of epithelial cells (transcellular or epithelial transport); other solutes move through junctions between epithelial cells (the paracellular pathway). Membrane transporters are not shown in this illustration.
Secretion
Transfer of molecules from extracellular fluid into lumen of nephron:
- dependent on membrane transport proteins to move organic compounds
- active process move substrates against concentration gradient & use secondary active transport to move into lumen
- secretion of K+ and H+ is important in homeostatic regulation
- enables nephron to enhance excretion of substance
- adds to substances collected during filtration, making excretion more effective
Fluid & Electrolyte Homeostasis
Water balance in the body
- Water makes up 50-60% of total body weight
- main entry of water is through food & drink
- most lost water in urine
- homeostasis maintains water balance unless there is pathology or an abnormal ingestion of water
Kidneys in water balance
- Kidneys cannot replenish lost water; only preserve or get rid of excess amounts
- volume loss replaced from the environment
- renal filtration will stop if there is a major loss causing extremely low blood pressure and blood volume
Urine concentration
- Osmolarity of urine measure of how much water is excreted by kidneys
- osmolarity changes as filtrate flows through nephron
- Diuresis - removal of excess water in urine
- diuretics: drugs that promote urine excretion
- Kidney controls urine concentration
- varying amounts of water and Na reabsorbed in distal nephron (distal tubule & collecting duct)
Osmolarity changes through nephron
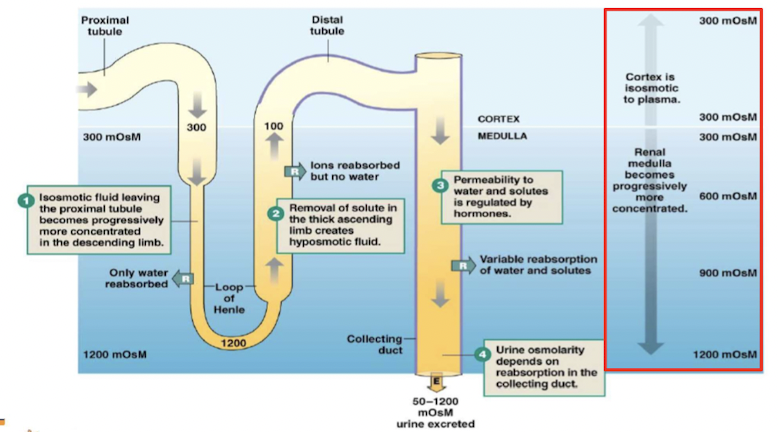
Countercurrent multiplier system
Exchange is enhanced by active transport of solutes
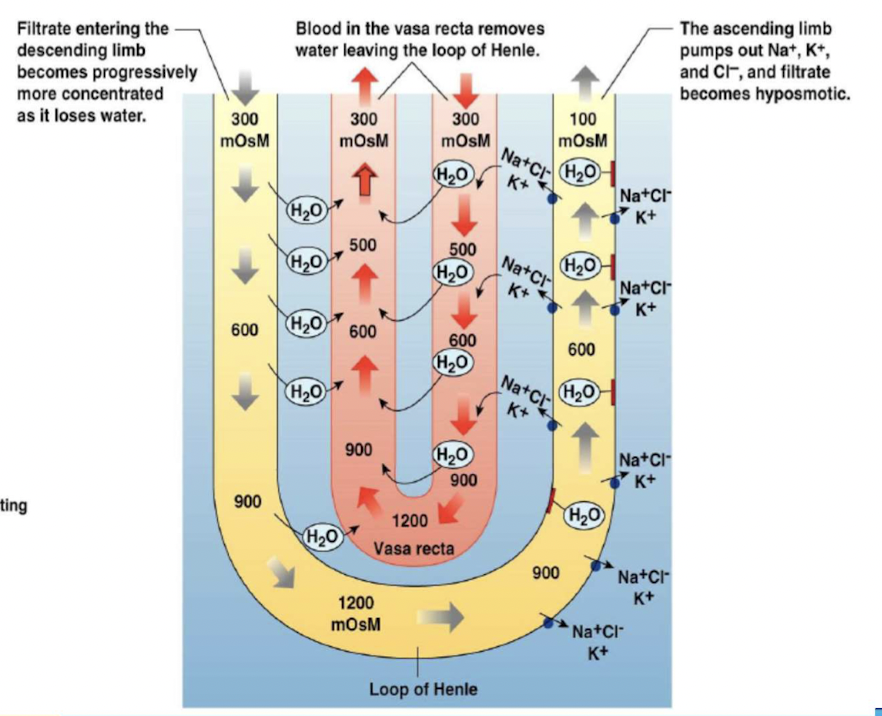
- Two components:
- loops of Henle that leave the cortex, dip down into the more concentrated environment of the medulla, then ascend into the cortex again.
- peritubular capillaries - vasa recta, also forming hairpin loops.
Water reabsorption
Distal tubule and collecting duct cells alter permeability to water
process involves adding or removing water pores (aquaporins) in apical membrane
depends on secretion of vasopressin/antidiuretic hormone (ADH)
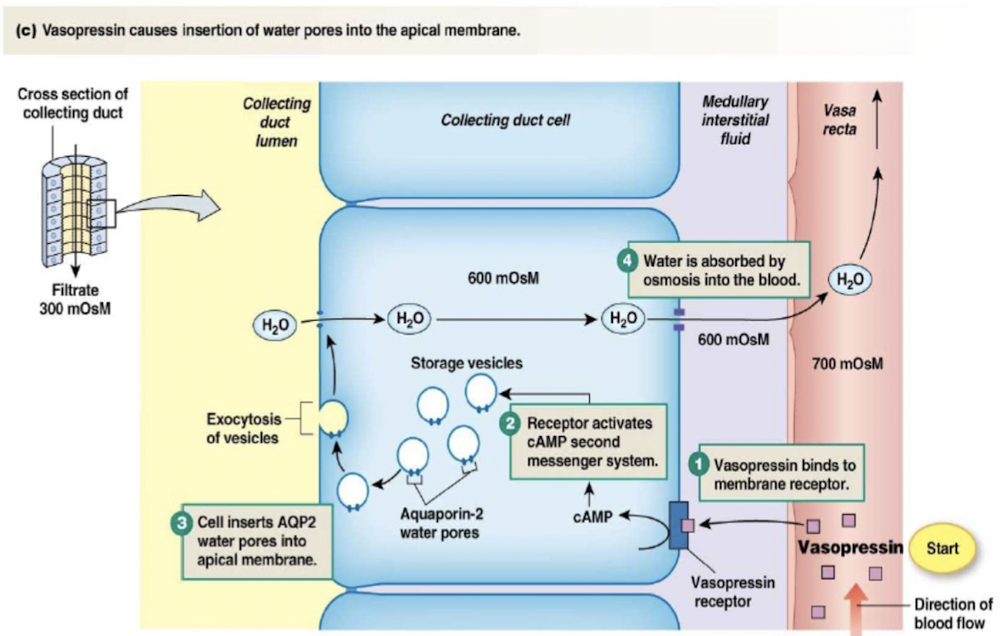
Control of vasopressin secretion
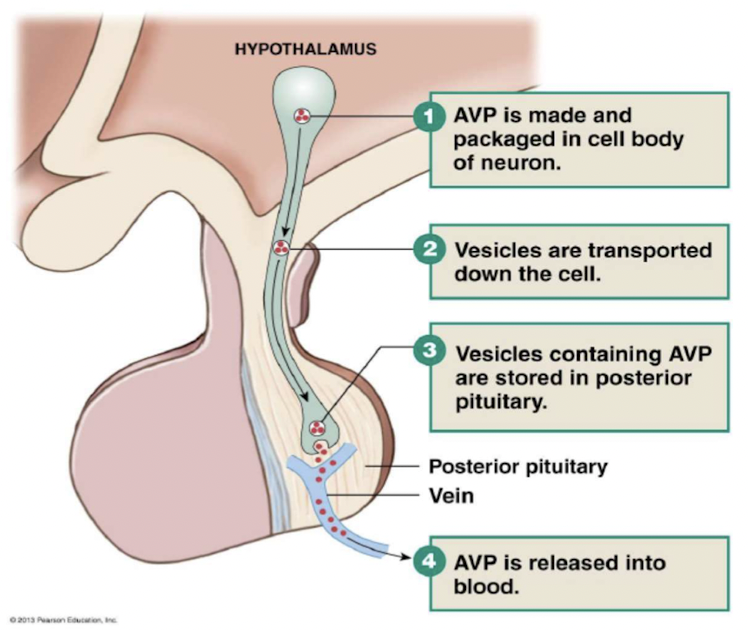
3 stimuli controlling vasopressin secretion:
- {{Plasma osmolarity > 280mOsM{{
- higher the osmolarity, more vasopressin released by posterior pituitary
- osmoreceptors in hypothalamus detect changes in osmolarity
- {{Blood pressure{{
- {{Blood volume{{
Renin-Angiotensis-Aldosterone System (RAAS)
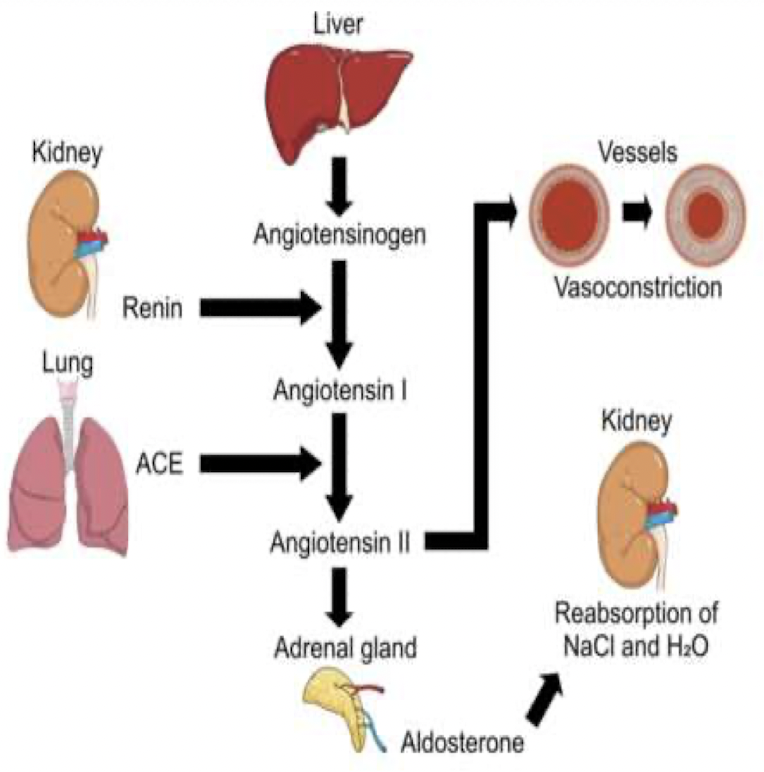
- Angiotensis II (ANG II) is the usual signal controlling aldosterone release from the adrenal cortex
- The RAS pathway begins when afferent arterioles secrete renin
- Renin converts inactive angiotensinogen, into angiotensis I (ANG I)
- ANG I converted into ANG II by angiotensis-converting enzyme (ACE)
- ANG II → adrenal gland → synthesis and release of aldosterone
- Aldosterone reabsorb Na+ at collecting duct
ACE2 & SARS-CoV-2 (COVID-19 Virus)
- ACE2 is present in many cell types and tissues including the lungs, heart, blood vessels, kidneys, liver and gastrointestinal tract.
- ACE2 is highly abundant on type 2 penumocytes in alveoli
- When the SARS-CoV-2 virus binds to ACE2, it prevents ACE2 from performing its normal function to regulate ANG II signalling
- ANG II increases blood pressure and inflammation, death of cells in alveoli