Study Unit 9: Pathways That Harvest Chemical Energy
Lecture 9.1: Introduction to Cellular Respiration
Learning Outcomes:
Understand how energy from food oxidation (e.g., glucose) is coupled to ATP synthesis.
Write the reaction for the overall oxidation of glucose to carbon dioxide and water.
Recognize the importance of redox reactions in cellular respiration.
Name the cellular compartment for each stage of cellular respiration: glycolysis, pyruvate oxidation, citric acid cycle, and oxidative phosphorylation.
Describe the purpose of each stage.
Write an overview of the 10 reactions of glycolysis and list the net products.
Cells Harvest Chemical Energy from Glucose Oxidation
Cells obtain energy from glucose through metabolic pathways.
Five principles of metabolic pathways:
Complex transformations occur in a series of separate reactions.
Each reaction is catalyzed by a specific enzyme.
Many metabolic pathways are similar across organisms.
In eukaryotes, metabolic pathways are compartmentalized in specific organelles.
Key enzymes can be inhibited or activated to alter the pathway's rate.
Cellular Respiration Overview
Overall reaction:
(\Delta G = -686 \text{ kcal/mol})Three catabolic processes that harvest energy from glucose:
Glycolysis (anaerobic)
Cellular Respiration (aerobic)
Fermentation (anaerobic)
Redox Reactions in Energy Transfer
Energy release and transfer often involve electron transfer (redox reactions).
Electron transfer is associated with hydrogen ion transfer (dehydrogenation).
Coenzyme NAD^+ is a key electron carrier in redox reactions.
NAD^+ + 2H^+ + 2e^- \rightarrow NADH + H^+
Overview of Harvesting Energy from Glucose
Aerobic Conditions:
Oxygen (O_2) is the final electron acceptor.
Four metabolic pathways operate.
Anaerobic Conditions:
Pyruvate from glycolysis is metabolized by fermentation.
Glycolysis
Occurs in nearly all living cells.
Stepwise degradation of glucose and other monosaccharides.
Primarily an anaerobic process.
Glycolysis: glucose lysis or degradation.
Glycolysis Phases
Three phases:
Preparatory phase (investment phase, Steps 1-3):
Energy (ATP) is consumed.
Cleavage phase (Steps 4 & 5):
Glucose (6C) is split into two 3C phosphorylated molecules.
Oxidation and payoff phase (Steps 6-10):
NADH and ATP are produced.
Lecture 9.2: Glycolysis and Pyruvate Oxidation Learning Outcomes
Learning Outcomes:
Write an overview of the 10 reactions of the glycolytic pathway, and list the net products of the pathway.
Recognize the energy inputs and outputs of the glycolytic pathway and the reactions where substrate-level phosphorylation occurs.
Write down the pyruvate oxidation reaction (as a link between glycolysis and the citric acid cycle) and list the net products of the reaction.
Step 1: Phosphorylation of Glucose to Glucose 6-Phosphate
Free energy (G):
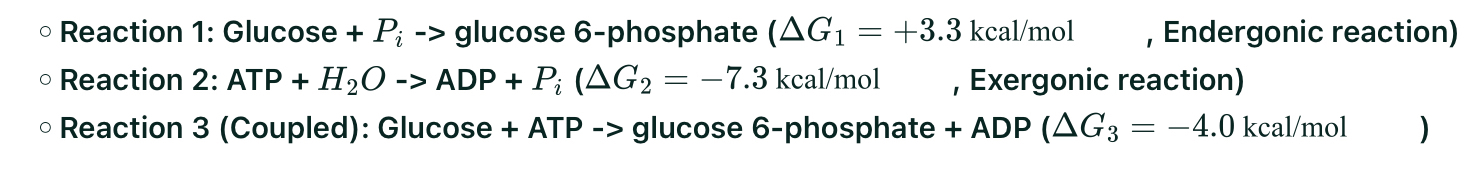
Formation of ATP by Substrate Level Phosphorylation
Two types of reactions:
Oxidation-reduction: Energy released by glucose oxidation is trapped by reducing NAD^+ to NADH.
Substrate-level phosphorylation: Energy released transfers a phosphate from the substrate to ADP, forming ATP.
Steps 6 & 7: Substrate Level Phosphorylation
Step 6:
Glyceraldehyde 3-phosphate + P_i + NAD^+ -> 1,3-Bisphosphoglycerate + NADH
Oxidation-reduction: Exergonic, energy trapped in NADH.
Step 7:
1,3-Bisphosphoglycerate + ADP -> 3-Phosphoglycerate + ATP
Substrate-level phosphorylation: Exergonic, energy trapped in ATP.
Step 10: Formation of ATP by Substrate Level Phosphorylation
Transfer of a phosphate group from PEP to ADP to yield ATP and pyruvate, catalyzed by pyruvate kinase.
Glycolytic Pathway Summary
Glucose is phosphorylated, degraded to 2 x 3C molecules, and oxidized to pyruvate.
2 ATP consuming steps (1, 3).
2 ATP yielding steps (7, 10).
Net products: Glucose + 2NAD^+ + 2ADP + 2Pi -> 2Pyruvate + 2NADH + 2H^+ + 2 ATP + 2H_2O
Pyruvate Oxidation
Pyruvate still contains chemical potential energy.
In the presence of oxygen, pyruvate is oxidized to CO2, NADH, and acetyl-CoA.
Reactions occur in the mitochondrial matrix.
Pyruvate Oxidation: Inputs and Outputs
For each glucose molecule:
2 Pyruvate + 2NAD^+ -> 2CO_2 + 2 Acetyl-CoA + 2NADH + 2H^+
Practice Questions
If reaction six of glycolysis is inhibited what will happen to the concentration of the glycolytic intermediates before and after the affected reaction?
Explain the importance of phosphorylation of D-glucose to glucose 6- phosphate.
Lecture 3: Learning Outcomes for Citric Acid Cycle
Write an overview of the 8 reactions of the citric acid cycle and list the net products of the cycle.
Identify the oxidation reactions of the citric acid cycle and recognize the release of the reduced electron carriers (NADH and FADH2) from these reactions.
Citric Acid Cycle (CAC)
Occurs in the mitochondrial matrix.
Acetyl CoA is the starting point.
Eight reactions completely oxidize the acetyl group to 2 molecules of CO_2.
Energy released is captured by GDP, NAD^+, and FAD.
Oxaloacetate is regenerated in the last step.
Also called the Krebs cycle (named after Sir Hans Krebs).
The CAC is the central pathway in metabolism.
CAC Completely Oxidizes Acetyl-CoA
Oxidation of acetyl-CoA produces the carbon dioxide we exhale.
Chemical potential energy stored in acetyl-CoA is transferred to NADH and FADH2.
GTP production results from substrate-level phosphorylation.
Step 1: Formation of Citrate
Condensation of acetyl-CoA with oxaloacetate to form citrate, catalyzed by citrate synthase.
Cycle activity depends on oxaloacetate concentration.
Steps 3 & 4: Oxidative Decarboxylation Reactions
Step 3: Oxidative decarboxylation of isocitrate (6C) to α-ketoglutarate (5C).
First reaction where CO_2 is released and NADH is produced.
Step 4: Oxidative decarboxylation of α-ketoglutarate (5C) to succinyl-CoA (4C).
Second reaction where CO_2 is released and second NADH is produced.
Step 5: Substrate Level Phosphorylation
Conversion of succinyl-CoA (4C) to succinate (4C).
Reaction couples hydrolysis of succinyl-CoA to the formation of GTP.
GTP can transfer a phosphate to ADP, converting it into ATP.
Step 6: Oxidation of Succinate to Fumarate
Succinate dehydrogenase catalyzes oxidation of succinate to fumarate.
Integral protein of the mitochondrial inner membrane in eukaryotes.
Complex II in the electron transport chain.
Third oxidation reaction, and FADH2 is produced.
Step 8: Oxidation of Malate to Oxaloacetate
Oxaloacetate (4C) is regenerated from the oxidation of malate (4C).
Final oxidation reaction, and third reaction where NADH is produced.
Net Products of the Citric Acid Cycle
Energy released by oxidation is conserved in the production of:
3 NADH
1 FADH2
1 GTP (ATP)
The energy of oxidations in the cycle is efficiently conserved.
Replenishment of Oxidized Electron Carriers
For the citric acid cycle to continue, acetyl CoA and oxidized electron carriers (NAD^+ and FAD) must be replenished.
The electron carriers are reduced and must be reoxidized.
If O2 is present, it accepts the electrons, and H2O is formed.
Practice Questions
Is it possible to get a net synthesis of oxaloacetate by adding acetyl-CoA to an extract of mitochondria that contains only the enzymes and cofactors of the citric acid cycle? Explain your answer.
Why is the citric acid cycle considered part of aerobic metabolism, even though molecular oxygen (O_2) does not appear in any of its reactions?
Lecture 4: Learning Outcomes for Oxidative Phosphorylation
Understand that oxidative phosphorylation consists of an electron transport chain and ATP synthase.
Consider the electron transfer system as a solid-state biological electric circuit powered by NADH and FADH2.
Understand how energy released from electron transport is coupled to ATP synthesis by explaining the electrochemical proton gradient process (chemiosmosis) that drives the ATP-synthase motor in the mitochondrion.
Distinguish between substrate-level phosphorylation and oxidative phosphorylation in cellular ATP formation.
Calculate the net ATP yield from the complete oxidation of n (a given number of) glucose molecules to CO2 and H2O.
Oxidative Phosphorylation
High-energy electron carriers (NADH and FADH2) converge at the electron transport chain (ETC).
NADH and FADH2 are not oxidized directly by O_2, but via several oxidation-reduction reactions catalyzed by electron carriers that make up the respiratory chain (ETC).
Free energy is released as electrons are transferred along the ETC from an electron donor (NADH and FADH2) to an electron acceptor (O_2).
ETC is coupled to oxidative phosphorylation - the redox reactions of the ETC convert the energy in the electrons to potential energy used to synthesize ATP.
Electron Transport Chain (ETC)
Electron transport is carried out by 4 closely related membrane-bound protein complexes and 2 electron carriers: Ubiquinone (Q) and cytochrome c.
Chemiosmotic Mechanism
Electrons (carried by NADH and FADH2) from glycolysis and the citric acid cycle feed the electron carriers of the inner mitochondrial membrane, which transfer protons (H^+) out of the matrix to the intermembrane space.
Proton transfer creates an imbalance of H^+ and a charge difference between the intermembrane space and the matrix. This imbalance is the proton-motive force.
The proton-motive force drives protons back to the matrix through the H^+ channel of ATP synthase (the F0 unit). This movement of protons is coupled to the formation of ATP in the F1 unit.
ATP Synthase
Molecular motor with two parts:
F_0 unit: transmembrane H^+ channel.
F_1 unit: projects into the matrix; rotates to expose active sites for ATP synthesis.
ATP synthase is the same in all living organisms.
Oxidative Phosphorylation Forms ATP
When H^+ diffuses through the channel, potential energy is converted to kinetic energy, causing the central polypeptide to rotate.
Energy transmits to the catalytic subunits of F_1, resulting in ATP synthesis (converting ADP and Pi to ATP).
Theoretical ATP Yield
Electron Carrier | Number of H^+ Transported | Number of H^+ Needed for ATP Synthesis | Number of ATP Produced |
|---|---|---|---|
NADH | 10 | 4 | 10/4 = 2.5 ATP/NADH mol |
FADH2 | 6 | 4 | 6/4 = 1.5 ATP/FADH2 mol |
Net product of 32 ATP (4 by SLP & 28 by OxP) results from oxidation of one glucose molecule.
SLP: Substrate Level Phosphorylation; OxP: Oxidative Phosphorylation.
Lecture 5: Fermentation, Metabolic Regulation, and Comparison of Respiration & Photosynthesis
Learning Outcomes
Describe the glucose fermentation process (to lactic acid or ethanol) and its biological significance.
Recognize that the breakdown products from the degradation of carbohydrates, proteins, and lipids converge on the citric acid cycle to produce ATP.
Explain how cellular respiration is regulated by energy levels of the cell.
Compare and contrast respiration and photosynthesis. Focus on ATP, water, CO_2, and high-energy electron carriers NADH or NADPH in the two processes.
Cellular Respiration in the Absence of Oxygen
Cellular respiration can occur in the absence of oxygen (anaerobic respiration), but it needs a final electron acceptor.
Many bacteria and archaea use alternate electron acceptors such as SO4^{-2}, NO3^{-} , Fe^{3+}, and CO_2.
The electron transport chain in these bacteria is located in the plasma membrane, not in an internal membrane.
Fermentation
In the absence of oxygen, some energy is harvested from glucose.
Pyruvate produced from glycolysis can be reduced to lactic acid or ethanol (alcohol).
Cellular respiration yields much more energy than fermentation.
Lactic Acid Fermentation
Occurs in animal, human, and some bacteria cells.
In the absence of oxygen, pyruvate produced is reduced to lactic acid.
This regenerates NAD^+.
The NAD^+ can then be reduced in glycolysis, and ATP is still synthesized in small amounts for use by the cell.
Glucose + 2ADP + 2Pi 2NADH -> 2 lactate + 2ATP + 2NAD^+
Ethanol Fermentation
Occurs in yeasts (fungi) and some plant cells.
Pyruvate releases carbon dioxide, and the acetaldehyde produced is reduced to form ethanol.
This regenerates NAD^+ so that ATP can be generated during glycolysis.
Used to produce alcoholic beverages.
Glucose + 2ADP + 2Pi 2NADH -> 2 ethanol + 2ATP + 2NAD^+
Relationships Among Major Metabolic Pathways
Fatty acids and amino acids are also useful sources of energy.
Metabolic Pathways Are Interrelated and Regulated
Pathways that generate ATP can be slowed depending on the levels of free energy available in the cell.
Indicators of energy available to the cell are concentrations of ATP, NAD^+, and NADH.
Allosteric Regulation
The main control point in glycolysis is phosphofructokinase (step 3), which is inhibited by ATP.
In fermentation, phosphofructokinase operates at a high rate to produce ATP.
If O_2 is present, more ATP is produced, which inhibits the enzyme and slows glycolysis.
The main control point in the citric acid cycle is isocitrate dehydrogenase (step 3).
It is inhibited by NADH and ATP; if too much of either accumulates, the citric acid cycle shuts down.