12-01: Thermochemistry
Energy
- A measure of the ability to work that is to move an object against an opposing force
- Can act on something chemically and release new products
- Energy comes in many forms: examples of which are heat, kinetic, light, sound, electricity, and chemical energy
Chemical energy is potential energy
- Potential energy: stored energy due to the position of an object
- Chemical potential energy: the energy due to the position of atoms relative to each other
- The farther objects are apart, the greater their potential difference
- The difference between reactant and product potential energy must be converted to or from another form of energy
Law of conservation of energy
- The total energy of the universe is constant - it can neither be created or destroyed ~ it can only be transformed
Thermal energy and heat
- Thermal energy: the total quantity of kinetic and potential energy in a substance
- Heat: the energy that transfers from one object to another when the two objects are at different temperatures and in some kind of contact (the transfer)
- Conduction: transferred by touch
- Convection: warning something that moves (e.g. air, water - cold water stays at the bottom and warm water rises because it is less dense -- convection cycle)
- Radiation: no medium transferring the heat
Temperature
A way of measuring potential thermal energy
Matter is composed of particles - particles are always in motion
Particles can move in 3 different ways
- vibrational motion
- rotational motion (typically involved in bonds)
- translational motion (monoatomics are only able to move like this)
Temperature is a %%measure of the average translational kinetic energy%%
Temperature is measured in either Celcius or Kelvins (K);
Kelvin = Celsius + 273.15
Temperature and average kinetic energy
Not all particles have the same amount of energy in a sample
The temperature of a substance represents an average of the kinetic energy that particles have
The diagram illustrates the warmer sample has more molecules of higher energy: this is called Maxwell-Boltzman distribution
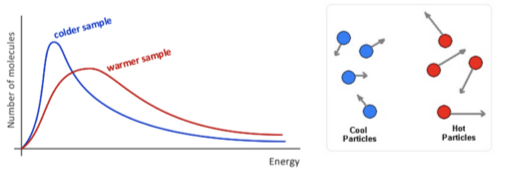 Colder sample holds less kinetic energy
Colder sample holds less kinetic energy
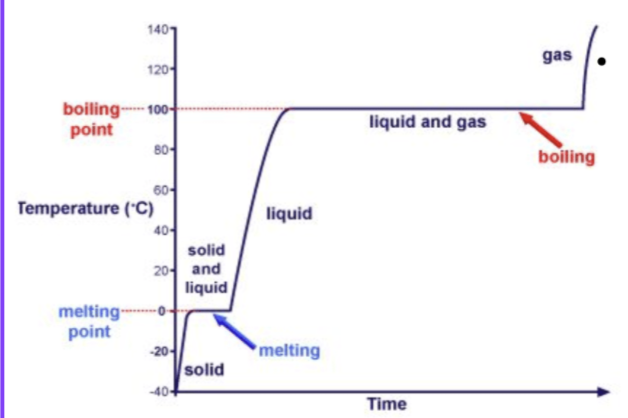
Energy transformations during changes of state
When solids, liquids and gases are heated, the particles move faster and farther apart
Since the added energy increases the speed of the particles the temperature rises
- ==Kinetic energy increases temperature==
During changes of state, ==the temperature remains constant and thus the kinetic energy of the particles does not change==
The added energy is converted to potential energy since it is used to separate (or bring together) particles and thus overcome intermolecular forces between them
- Potential energy causes a phase change
- When it is in the potential energy phase, it is getting rid of intermolecular forces
Units of energy
- Temperature - kelvins or celcius
- kelvin = celcius + 273.15
- Heat - joules
- 1kJ= 1000J
- Calorie: 1 cal is the energy needed to raise the temperature of 1g of water by 1ºC
- 1 cal = 4.184 J
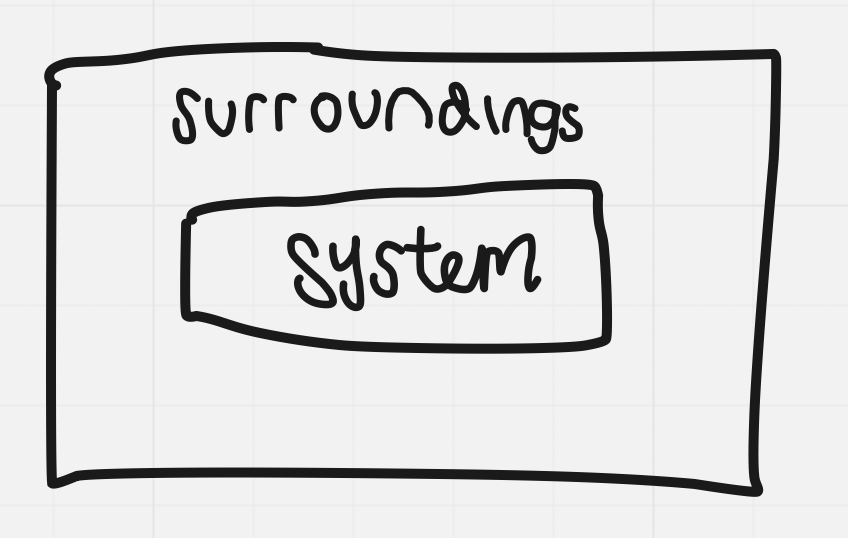
Systems and Surroundings
World is divided into a system and its surroundings
System: ^^the part of the world that we want to study^^ (e.g. a reaction mixture in a flask)
Surroundings: ^^everything else outside of the system^^
Types of systems:
Open system: can exchange both matter and energy with the surroundings (e.g. open reaction flask, rocket engine) - matter and energy can get out
Closed system: Can exchange only energy with surroundings, matter remains fixed (e.g. a sealed reaction flask)
Isolated system: can exchange neither energy nor matter with its surroundings (e.g. a thermos flask, a bomb calimeter)
Enthalpy (H)
==The amount of heat of a system is called enthalpy (H)==
@@The amount of heat of the surroundings is represented by the symbol q@@
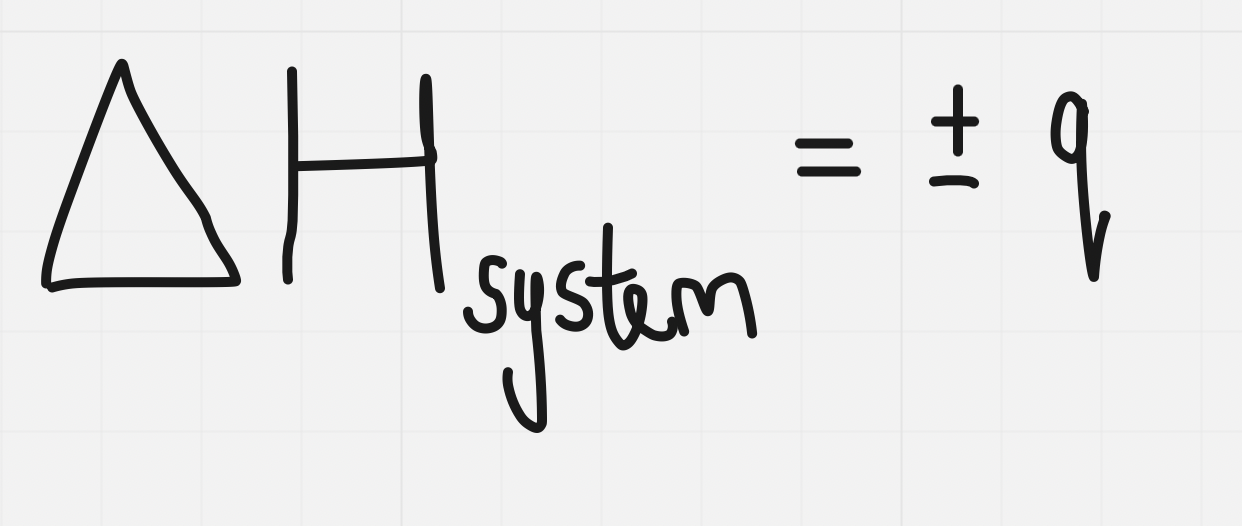 ∆H is the the enthalpy change - the energy absorbed from or released to the surroundigns during a chemical or physical change
∆H is the the enthalpy change - the energy absorbed from or released to the surroundigns during a chemical or physical changereaction is only valid at constant pressure
a teaspoon of boiling water has a higher temperature than a bathtub of boiling water but the water in the bathtub has more thermal energy in total and therefore can transfer more heat to a cooler object
Exothermic and endothermic reactions
Exothermic process: a change that releases energy to the surroundings
- Given a negative sign as the system is decreasing
- Release of heat = decrease in enthalpy
- ∆H<0
^^-∆H = +q^^
Endothermic process: a change (e.g. a chemical reactiojn that requires or absorbs energy from the surroundings
- Given a positive sign as the system is increasing
- Input of heat corresponds to an increase in enthalpy
- ∆H > 0
^^+∆H = -q^^
Measuring heat
- Temperature is the average kinetic energy of the particles in a sample of matter
- Exothermic reaction: heat given off, temperature of water rises
- Endothermic reaction: heat taken in, temperature of water drops
Heat capacity
- The more modes of motion a particle has, the more energy it can absorb without the temperature changing as much
- This is called the heat capacity of a substance
- Metals have a low heat capacity
Specific Heat Capacity
Specific heat capacity (c): the quantity of thermal energy required to raise 1g of a substance by 1ºC
More heat required to raise the temperature of a large sample of a substance than is needed for a smaller substance
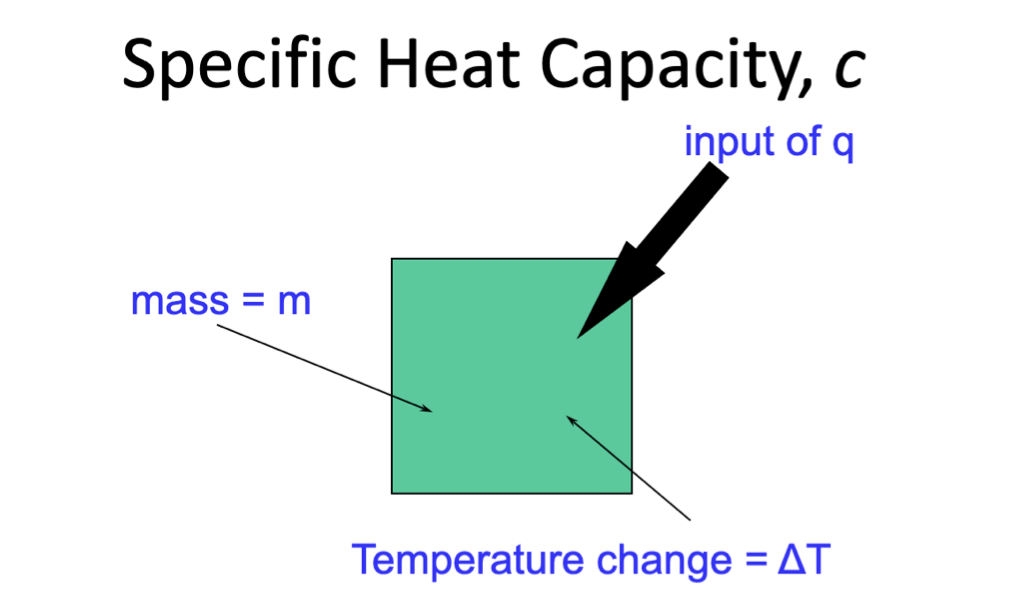
q=the total amount of thermal energy absorbed/released by a chemical system
The magnitude of q tells you how much energy is involved - the sign tells you about the direction of energy transfer
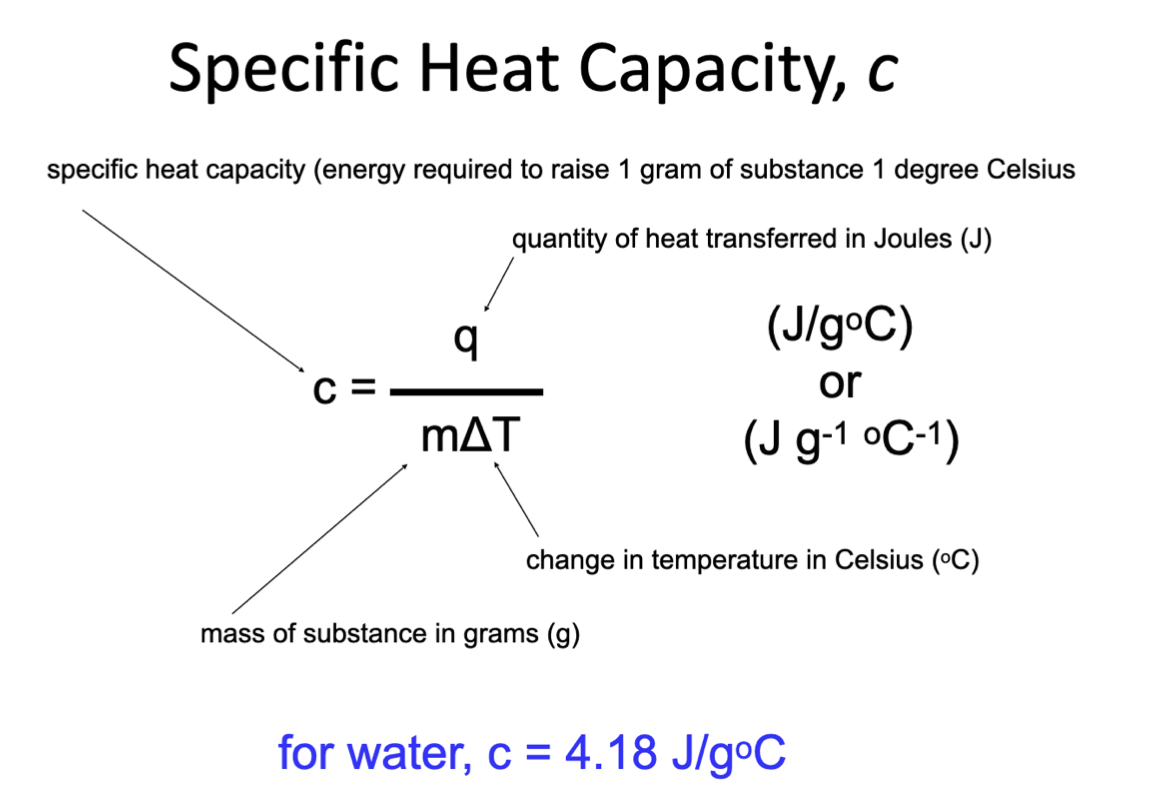
Specific heat capacities
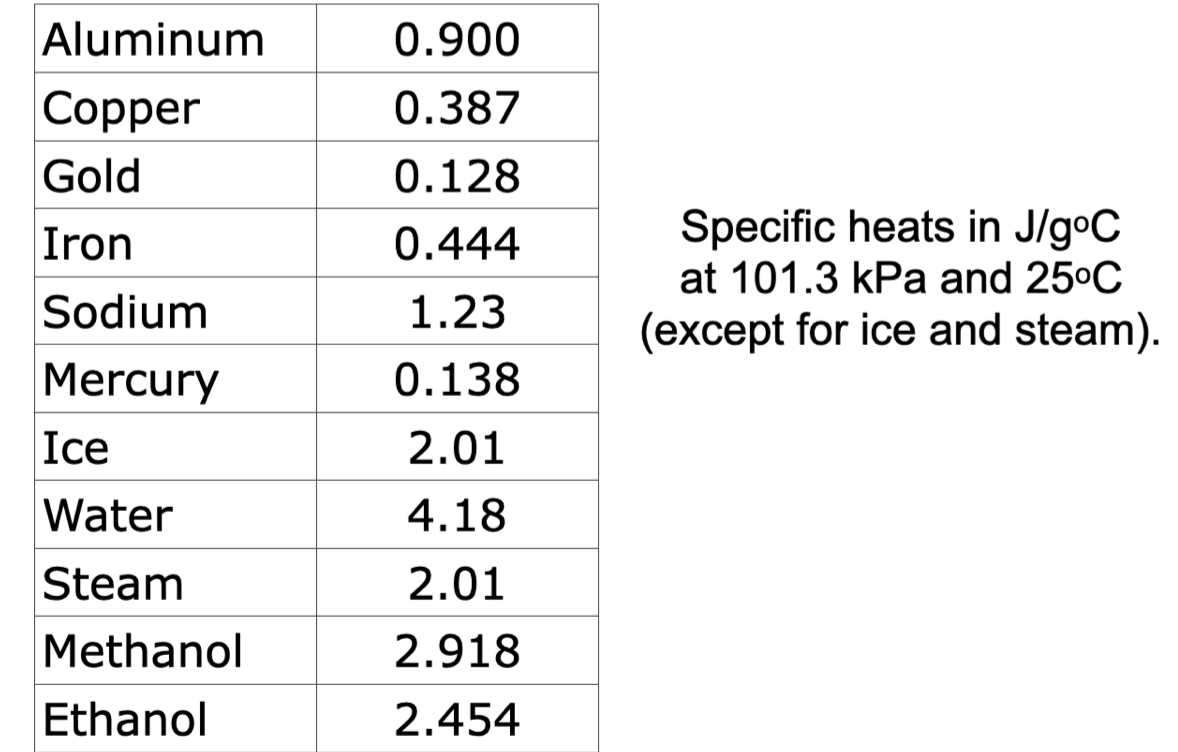 q = mc∆T
q = mc∆T
- The specific heat capacity of a substance can be used along with the mass and temperature change of a substance to determine the amount of heat energy (q) transferred
- Use GRASP conventions when solving
Calorimetry and enthalpy
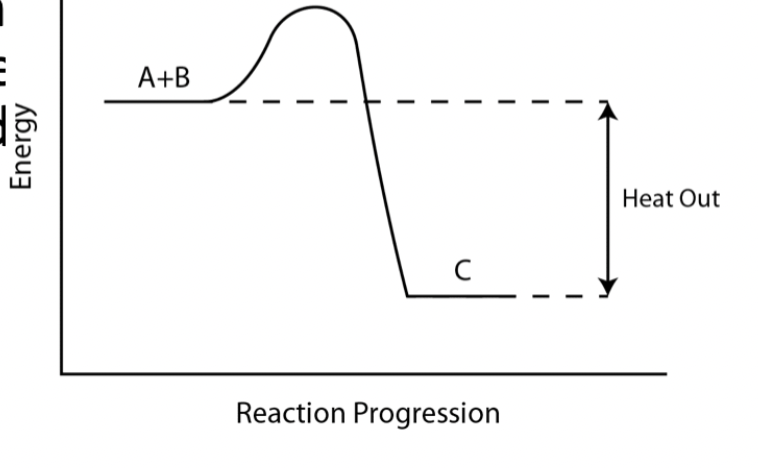
Exothermic reactions
Exothermic reactions: energy is released from the system into the surroundings - this means that some of the potential energy of the reactants was changed into kinetic energy which heats the surroundings
 Endothermic reactions
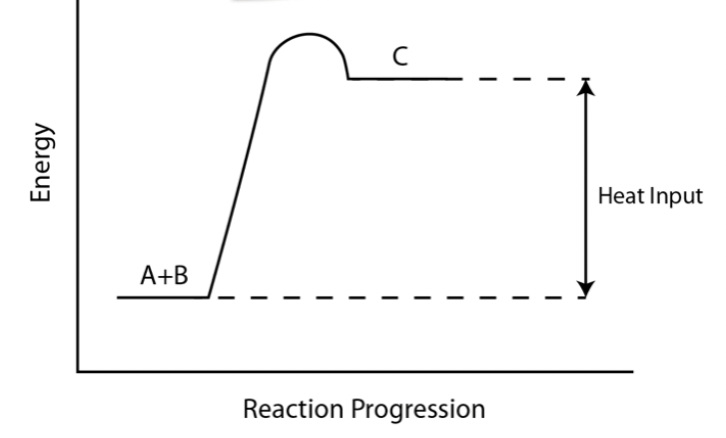
Endothermic reactions
Endothermic reactions: thermal energy from the surroundings is absorbed and increases the potential energy of the system
Calorimetry
- A calorimeter: device that is used to measure the energy changes in the surroundings during a physical or chemical change
- Original unit of heat energy is the calorie
- 1 cal = 4.18 J
Assumptions we make
- No heat is transferred between calorimeter and outside the environment, assume no loss
- Any heat absorbed or released by calorimeter itself is negligible
- Dilute aqueous solutions are assumed to have the density and specific heat capacity of pure water
- density of water = 1.0g/mL
- specific heat capacity of water = 4.18 J/gºC
Enthalpy change
- The calorimetry process can be used to determine the change in potential energy of the compounds in a chemical system
- this is called the enthalpy change, it’s given the symbol ∆H
The enthalpy change of the system will be = to but opposite sign to the heat absorbed by the energy absorbed by the surroundings
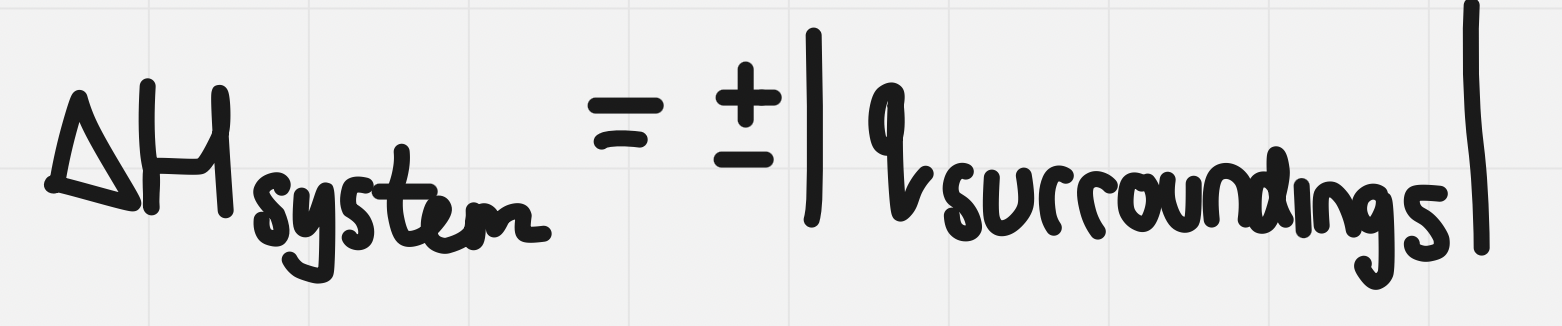
Representing Enthalpy Changes
- Thermochemical equations with energy terms
- If energy is with the reactants - endothermic
- If energy is with the products - exothermic
- Thermochemical equations with ∆H values
- ∆H = a number value
- The number value’s sign dictates whether it is endothermic or exothermic - if the value is positive then it is endothermic, if the value is negative then it is exothermic
- When an equation is balanced and has the coefficients these are molar ratios. This means that you can divide them out but then you also must divide your enthalpy
Example of this:
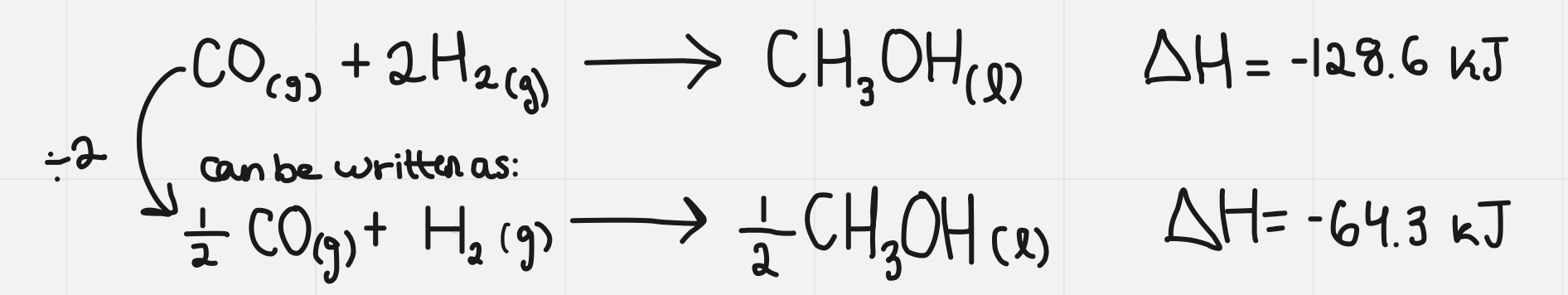
- Molar enthalpy of reaction
Write the kind of reaction as a subscript
e.g. for cellular respiration it would be
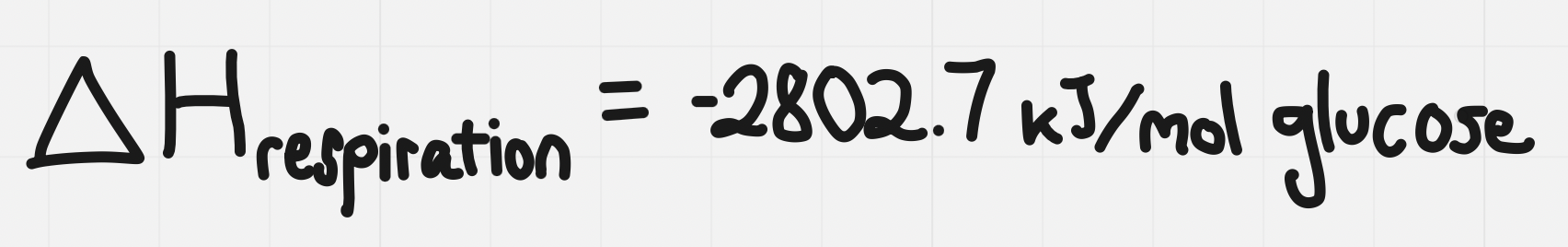 4) Potential Energy Diagram
4) Potential Energy Diagram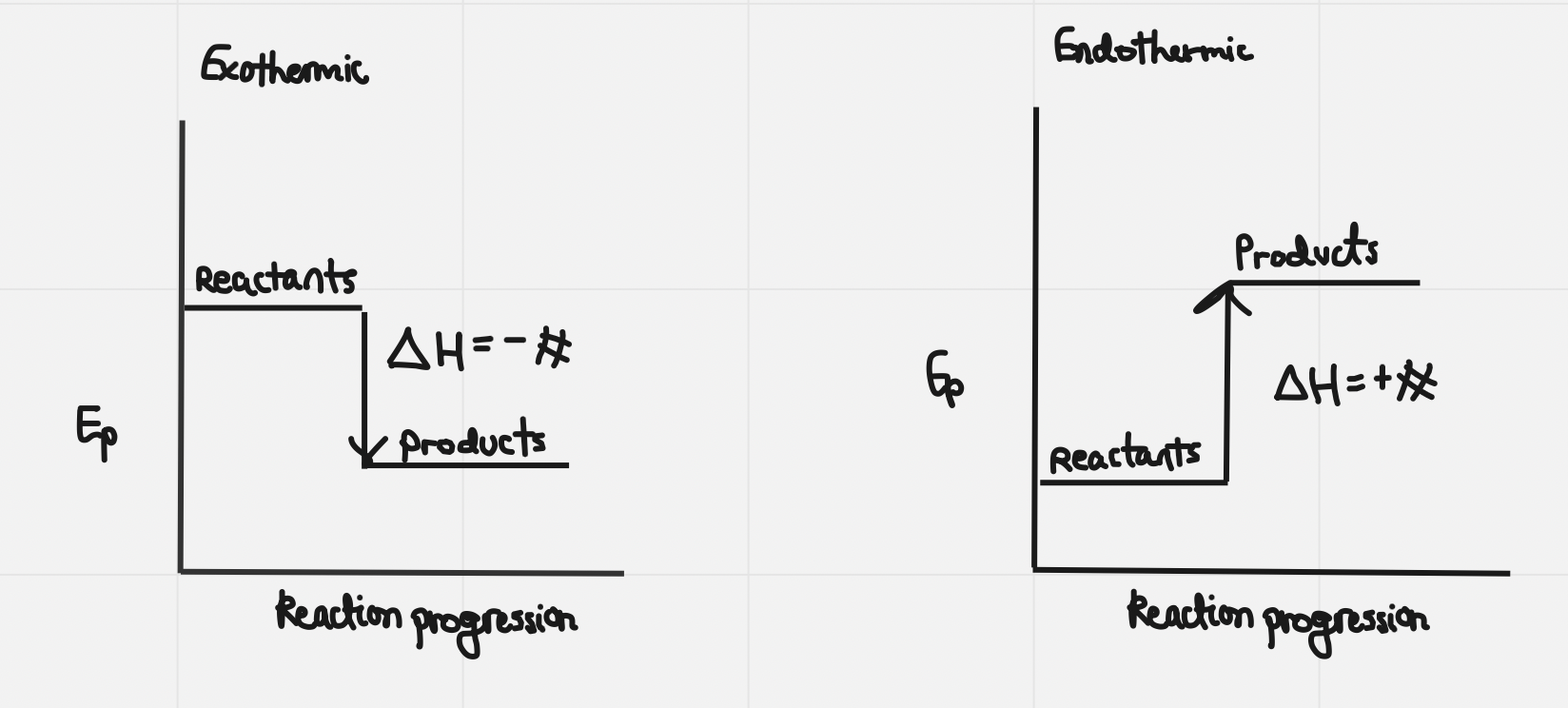
Enthalpies
- Enthalpy increases when heat energy comes in from the surroundings
- Enthalpy decreases when heat energy escapes/comes out into the surroundings
Enthalpy Change (∆H)
- What chemists study
- Energy absorbed from/released to the surroundings when a system changes from reactants to products (i.e. a reaction occurs)
- Equal to the amount of heat transferred between the system and surroundings
- q and ∆H should be equal in magnitude, but their sign (which signifies direction, either in or out) will be different
Enthalpy symbols
Any change involving energy will have an enthalpy associated with it
- Physical or chemical change - what process is occurring?
- Standard values will be for one mole of reactant under conditions such as STP and SATP
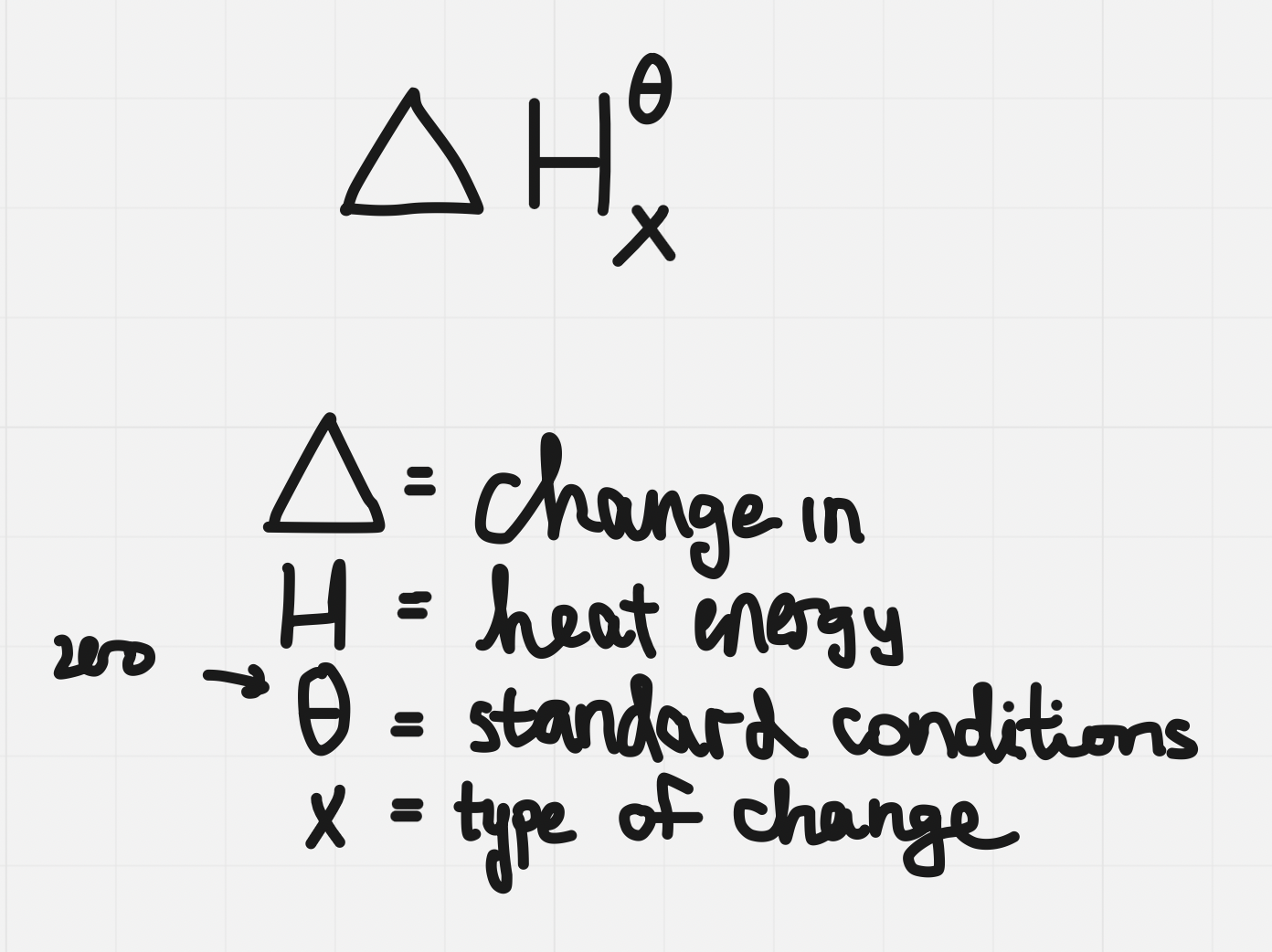
Sign matters in enthalpy change
- Enthalpy change for @@exothermic reactions are given a negative sign@@ (∆H<0)
- heat is released from the system
- temperature of the surroundings (q) increase
- Enthalpy change for ==endothermic reactions are given a positive sign ==(∆H>0)
- heat is absorbed by the system
- temperature of the surroundings decrease
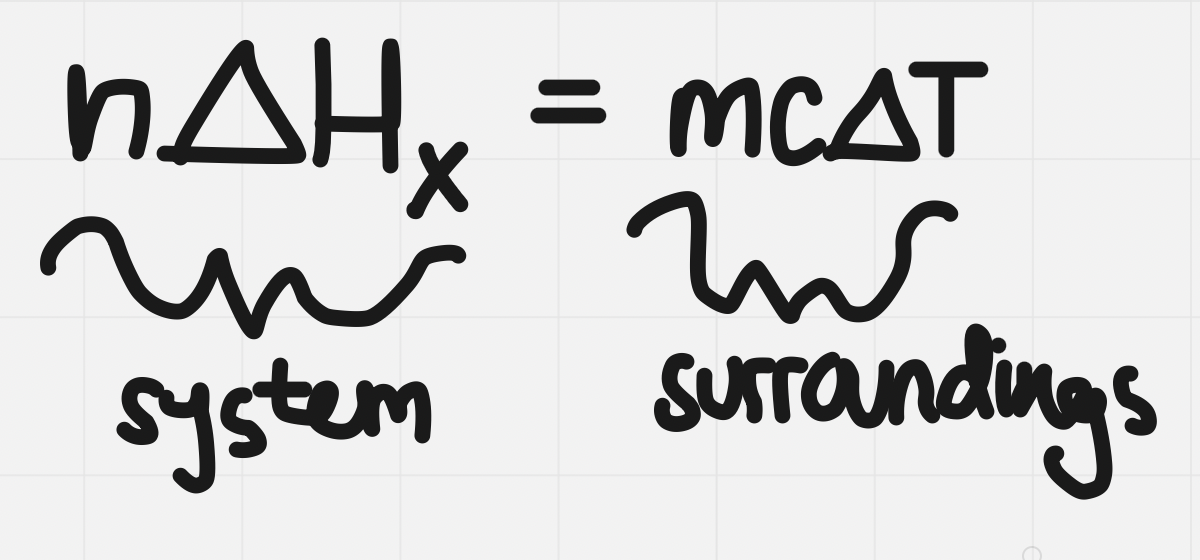
Molar enthalpy
- Molar enthalpy: enthalpy change associated with a physical, chemical, or nuclear change involving @@one mole@@ of a substance
- always convert grams to moles
- Represented by ∆H^^ₓ^^ where ^^x^^ is a letter or combination of letters which indicate the type of change that is occurring
e.g.
 To calculate ∆H for some amount of substance other than a mole:
To calculate ∆H for some amount of substance other than a mole:
 Example:
Example:

- The experimental technique of measuring energy changes with an instrument called a calorimeter
- Used to obtain ∆Hₓ values (systems)
Energy changes
Law of conservation of energy
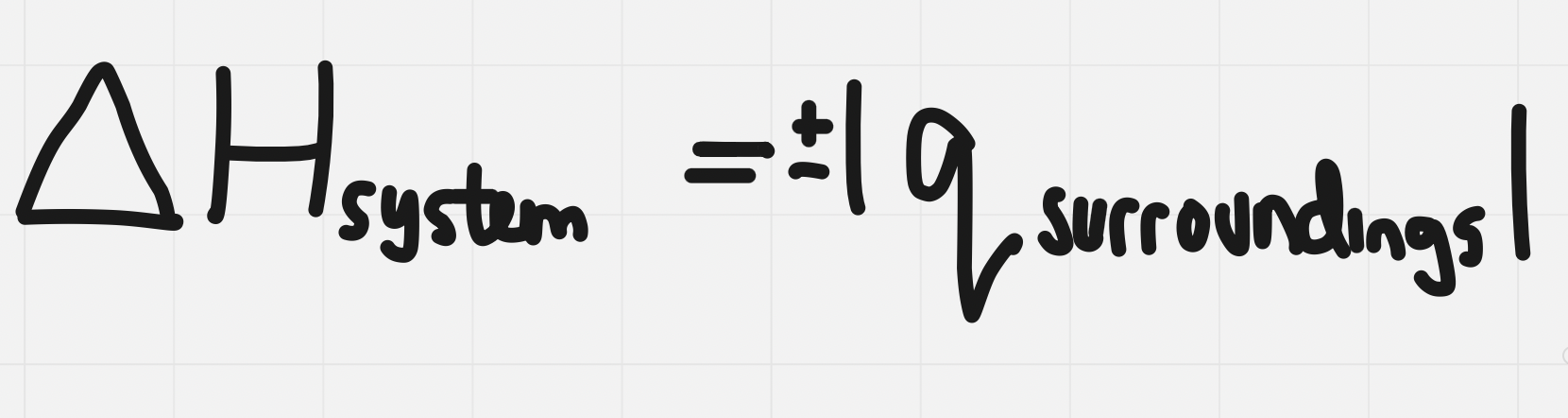 Combining this with
Combining this with

Leads us to determine a formula to determine ∆Hₓ
Standard enthalpies of physical changes
- Enthalpy of solution: energy change from dissolving one mole of a substance
- Enthalpy of vaporization: energy change from converting one mole of liquid to gas
- Enthalpy of fusion: Energy change from converting one mole of solid to liquid
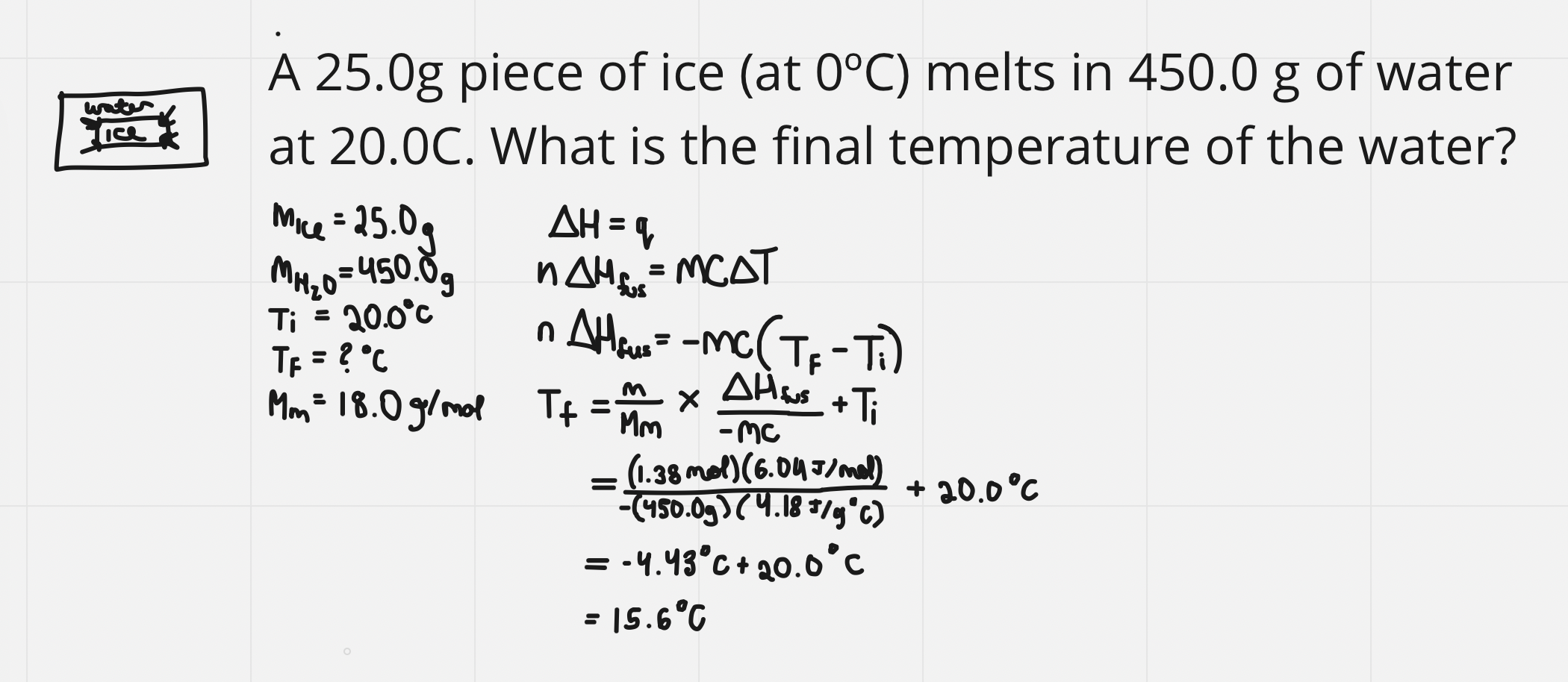
e.g. finding the enthalpy value
 e.g. using the enthalpy value
e.g. using the enthalpy value
Standard ethalpies of chemical change
- Enthalpy of combustion: energy change from burning one mole of substance
- Enthalpy of formation: energy change to produce one mole of substance from elements in standard state
- Enthalpy of neutralization: energy change for neutralizing one mole of acid or base per mole of water formed
e.g. finding enthalpy
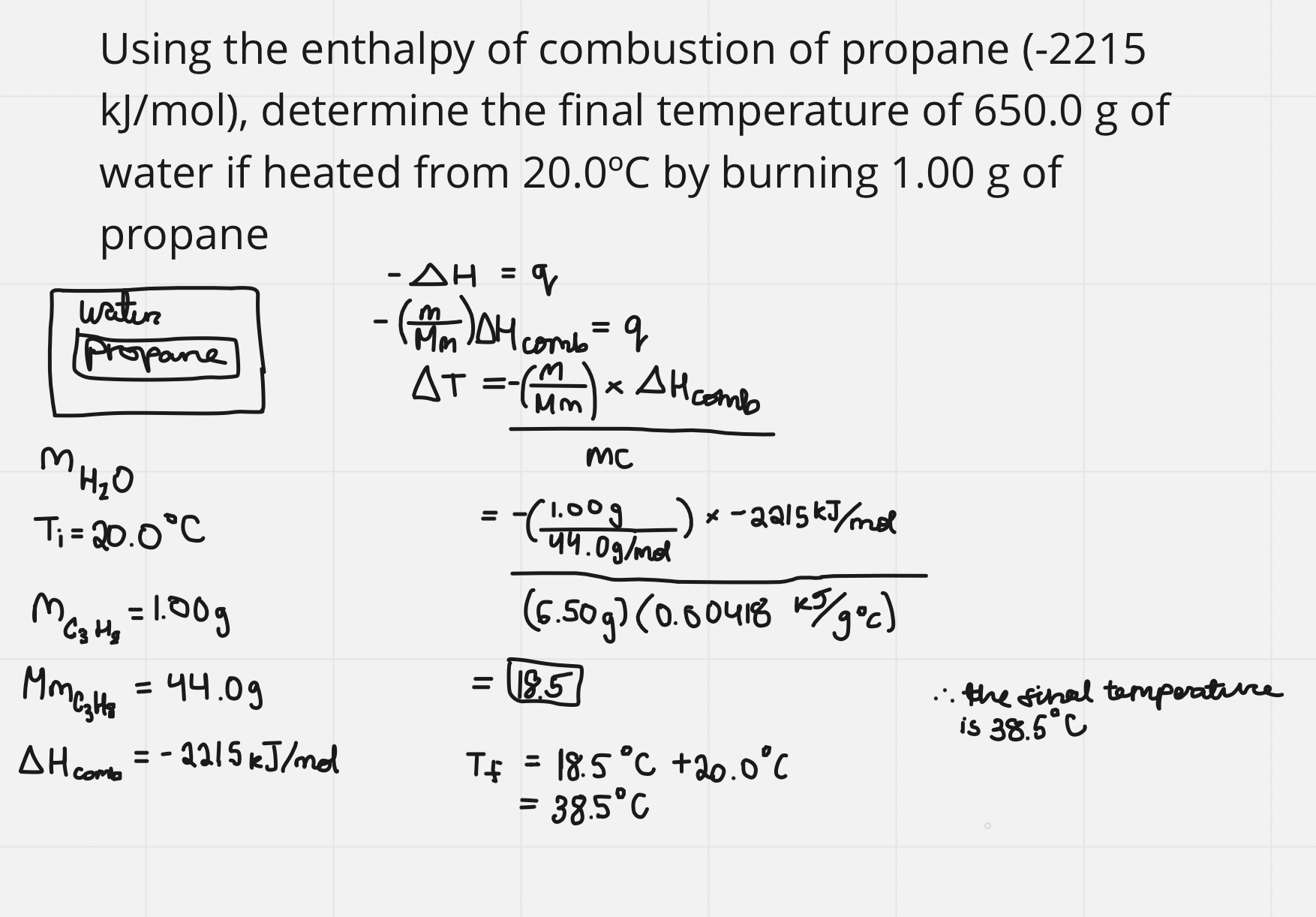
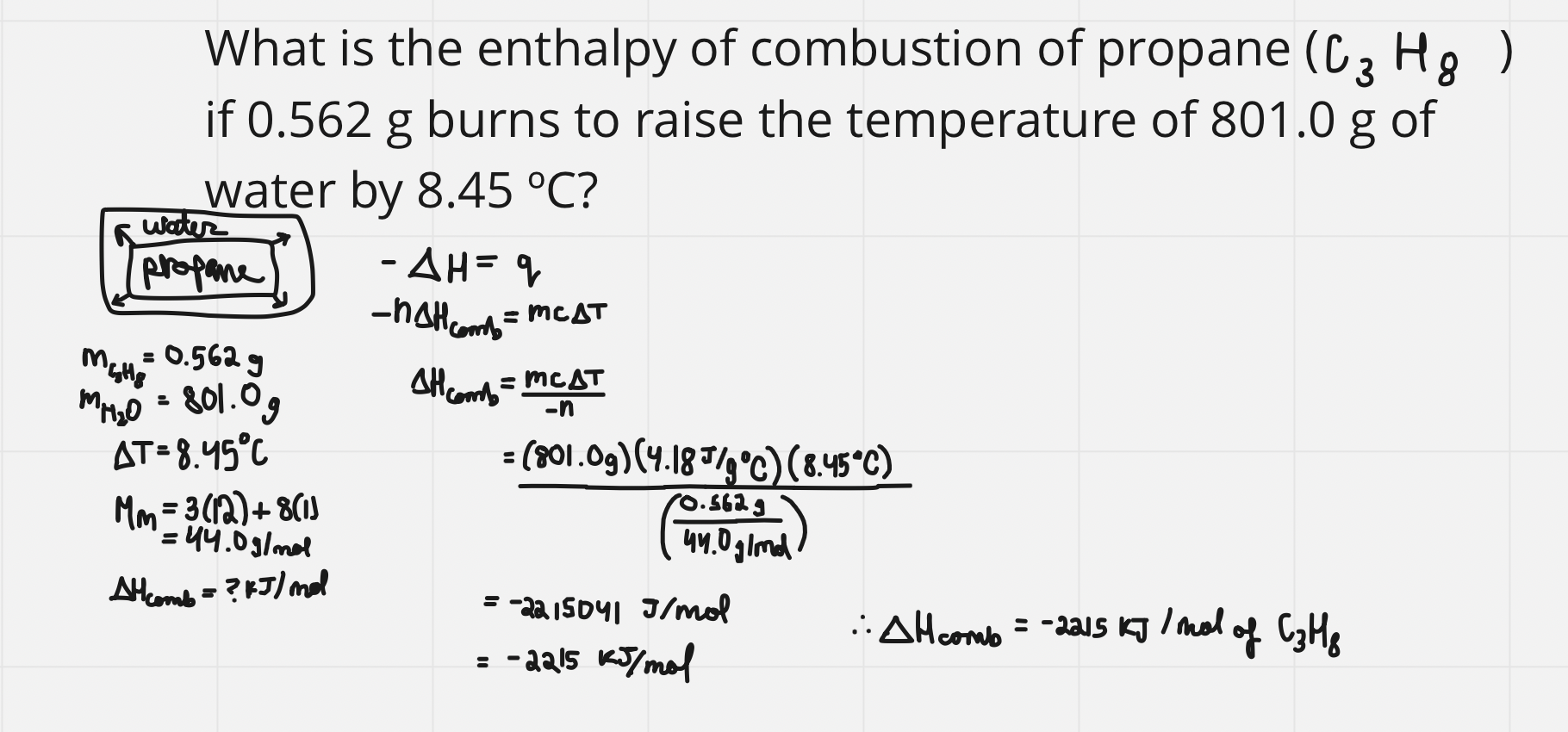 e.g. using enthalpy
e.g. using enthalpy
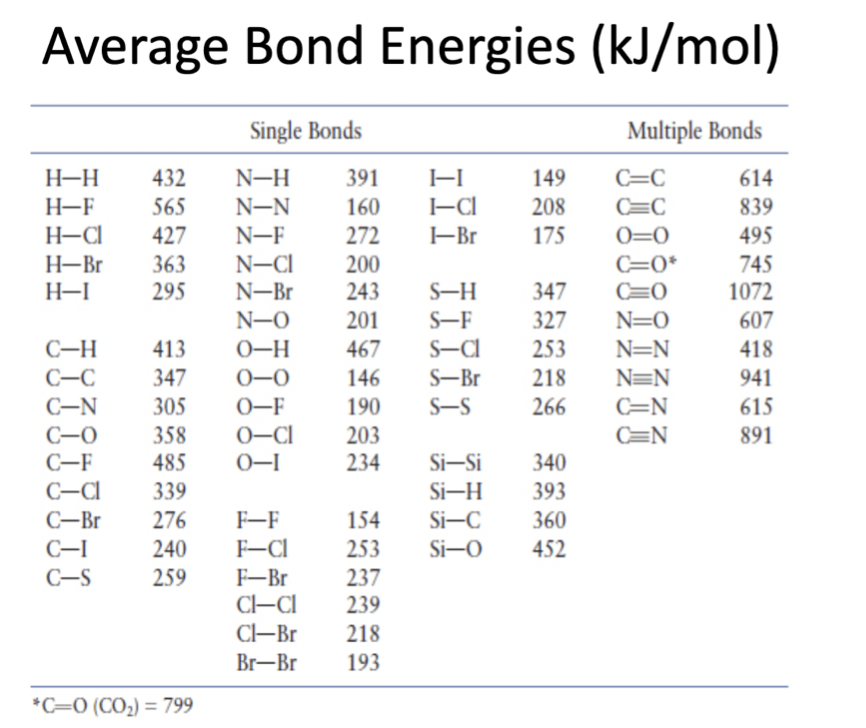
Bond energy
Energy is required when bonds are broken (endothermic)
Energy is released when new bonds form (exothermic
Same amount of energy to break and reform bonds
The amount of energy absorbed/released when breaking or forming a bond is the same
These energies are tabulated as an average - there are slight differences between bonds in different molecules
Reaction enthalpy from bond energies
- Every reaction can be thought of as endothermic when bonds are broken and exothermic when new bonds form
- The enthalpy change is determined from the degree of the exothermic and endothermic processes
Enthalpy of reaction
- @@Bond breaking is negative@@
- @@Bond forming will be positive@@
Enthalpy from bonds - formula
Σ (sigma) means “the sum of”
n is the amount (in moles) of a particular bond type
D is the bond energy per mole of bonds
If it is overall positive - endothermic
If it is overall negative - exothermic
Hess’s Law
State Functions
- Enthalpy change is a state function, meaning that the energy difference between 2 states (reactants and products) is the same regardless of how the change happened
State function properties
- Temperature
- Pressure
- Energy
- Volume
- Mass
- These are state function properties because their values don’t rely on the path
Constant heat summation
Hess’s law states that the enthalpy change of a reaction is equal to the sum of the enthalpies of all the steps required for the reaction to happen
Similar to adding vectors
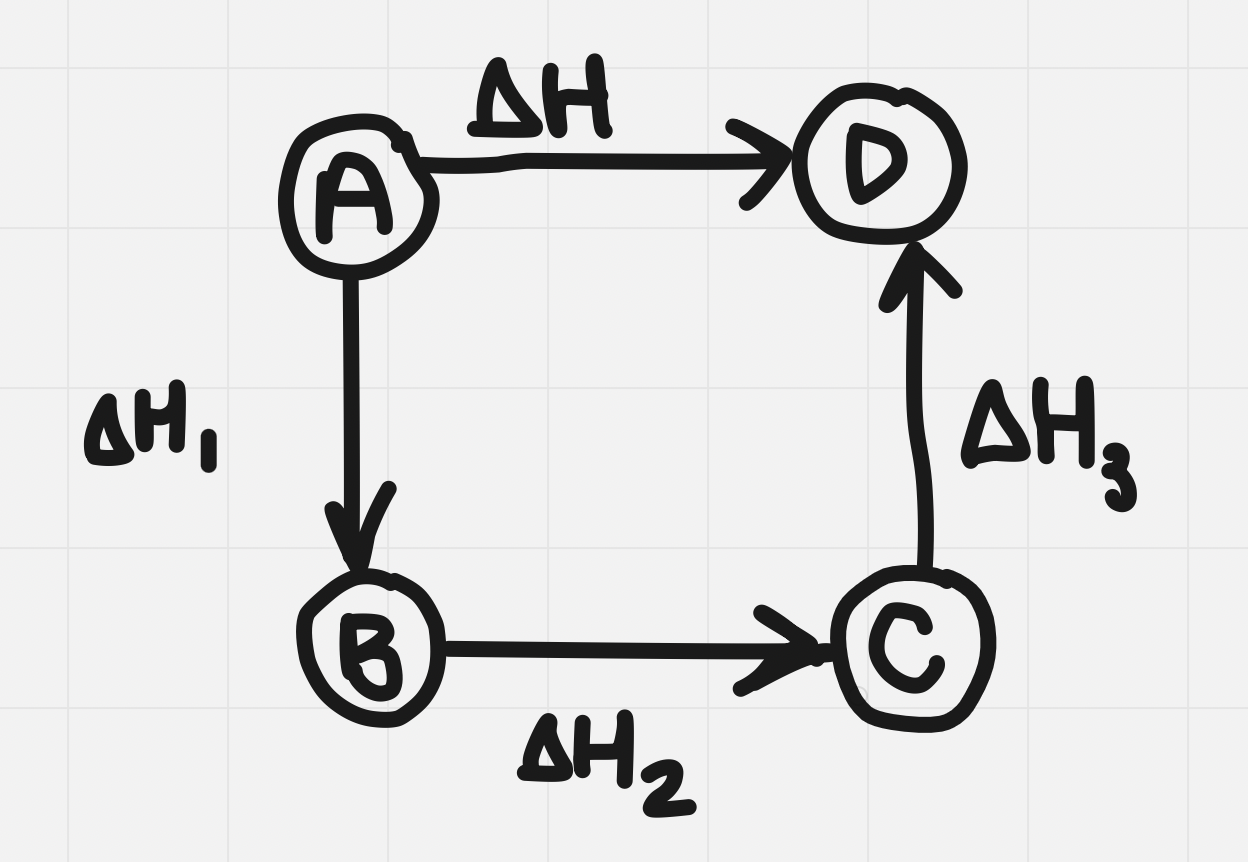
Hess’s law
- Since determining the enthalpy of a reaction by calorimetry is not always possible, Hess’s law can be used to determine unknown reaction enthalpies from known ones
Adding equations
When adding equations, cancel out the substances found on both sides of the arrow
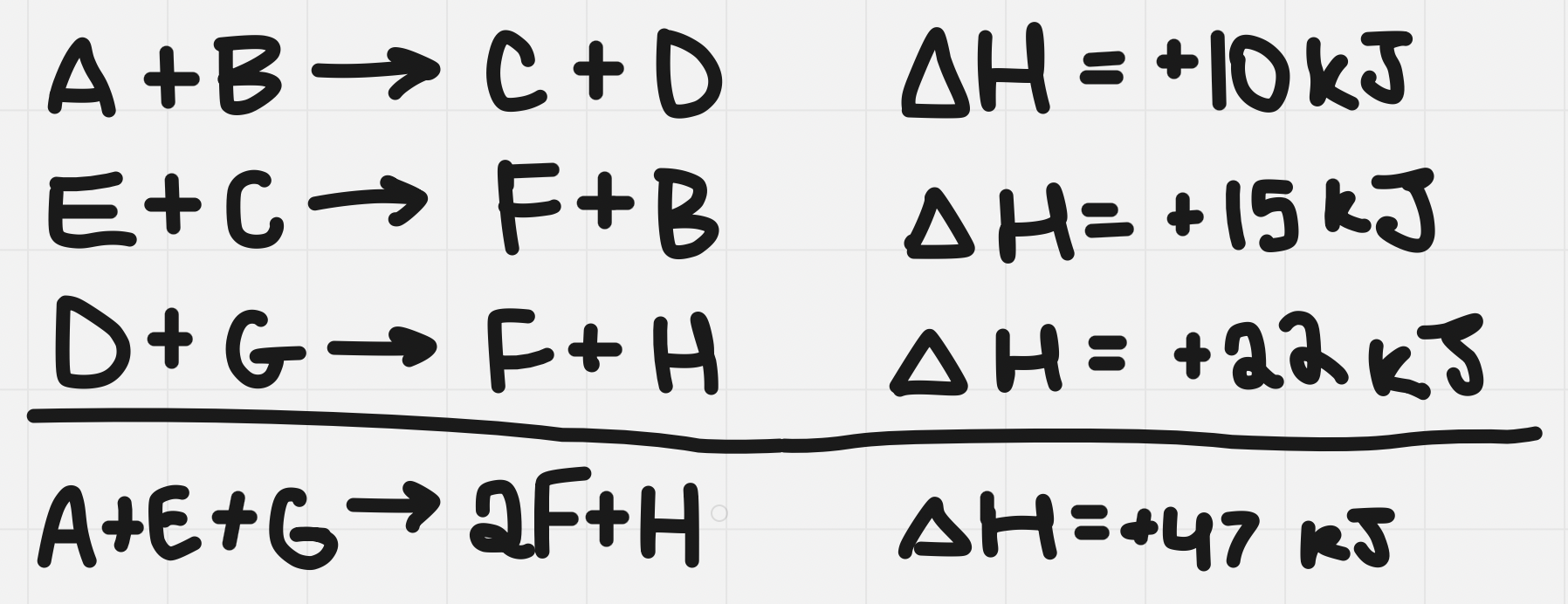
Manipulating Equations
- Before you add, you can multiply/divide/flip equations
- You must do the same thing to the enthalpy - if flipping equation then flip ∆H’s sign (+ to - or - to +)
Formation Reactions
Reaction that produces one mole of a substance from elements in their standard state
ONE mole of product (often fractions needed to balance)
Elements in standard state - see periodic table (red = gas, black = solid, blue = liquid)
Formation reaction for water:
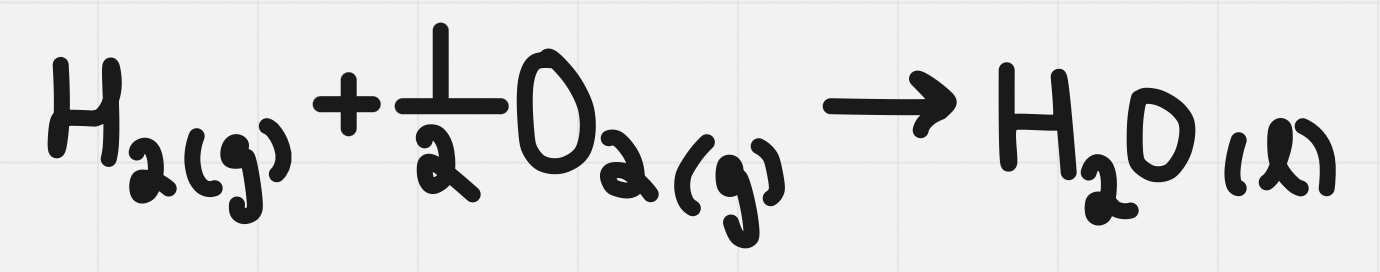
Enthalpy of formation
The tabulated enthalpy values for the formation of compounds can be helpful for determining the enthalpy of other reactions
elements in standard state (by themselves) have an enthalpy of formation of zero
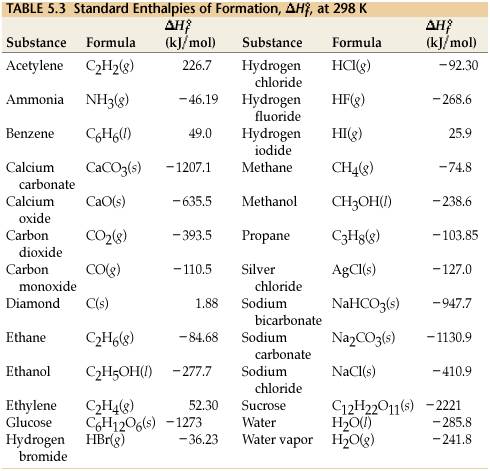
Using formation reactions
- Each reaction is a reactant un-forming into elements and then products being formed
- Using Hess’s law, you can solve for the enthalpy of reaction from enthalpy of formation
Formation reaction formula
A quicker method of finding the enthalpy of reaction is with the following formula
Don’t forget to multiply by the moles of each reactant or product in the reaction