reactions
Topics include:
Balancing chemical equations
Adjusting the coefficients of reactants and products to ensure an equal number of atoms on both sides
MINHO:
Metals: Balance any metals present in the reactants and products.
Incorporate the coefficients: Add coefficients to balance the atoms of each element, making sure the number of atoms of each element is equal on both sides of the equation.
Non-metals: Balance the non-metals following the metals.
Hydrogen: Balance hydrogen atoms.
Oxygen: Balance oxygen atoms last.
compound equations
show all reactants and products in their complete form.
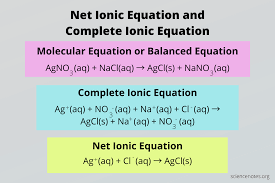
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
total ionic equations
Write all aqueous (aq) substances as their dissociated ions.
Leave solids (s), liquids (l), and gases (g) as they are.
Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq)
Step by Step Guide:
1. Start with a Balanced Molecular Equation:
Ensure the chemical equation is balanced, meaning the number of atoms of each element is the same on both sides of the equation.
2. Identify Aqueous Substances:
Determine which substances are in the aqueous (aq) phase by looking for the (aq) state symbol.
If state symbols are not provided, you can use solubility rules to determine if ionic compounds are soluble and therefore aqueous.
Break Down Aqueous Substances into Ions:
Write down the ions that make up the aqueous substances, keeping in mind the charges of the ions.
Include the (aq) state symbol for each ion.
Example: If you have NaCl(aq), break it down into Na+(aq) + Cl-(aq).
Do not break down solids, liquids, and gases into ions .
4. Write the Total Ionic Equation:
Rewrite the equation with the dissociated ions from the aqueous substances, keeping the non-aqueous substances as they were in the molecular equation.
Example: If the molecular equation is NaCl(aq) + AgNO3(aq) -> AgCl(s) + NaNO3(aq), the total ionic equation is: Na+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) -> AgCl(s) + Na+(aq) + NO3-(aq
net ionic equations
identify spectator ions (appear unchanged on both sides) + eliminate them
Write down the remaining ions (and any solids/liquids/gases) to form the net ionic equation
EXAMPLE:
2KCl (aq) + AgNO3 (aq) → AgCl (s) + 2KNO3 (aq)
2K+ (aq) + 2Cl- (aq) + 2Ag+ (aq) + 2NO3- (aq) → 2AgCl(s) + 2K+ (aq) + 2NO3-(aq)
Step by Step Guide:
1. Write the Balanced Molecular Equation:
Start by writing the chemical equation in a balanced form, ensuring the number of atoms of each element is the same on both sides of the equation.
2. Identify Aqueous Substances:
Determine which substances are in the aqueous (aq) state (dissolved in water).
Soluble Ionic Compounds: Generally, compounds containing group 1A (Li, Na, K) and ammonium (NH4+) cations, nitrates (NO3-), and chlorides (Cl-), bromides (Br-), and iodides (I-) (except Ag+, Hg2+2, and Pb2+) are soluble and dissociate into ions in solution.
Strong Acids and Bases: Recognize common strong acids (HCl, HBr, HI, HNO3, H2SO4, and HClO4) and bases (group 1A hydroxides) as strong electrolytes that completely dissociate into ions.
3. Write the Complete Ionic Equation:
Rewrite the balanced molecular equation, but break down any aqueous substances into their constituent ions (include charges and the (aq) state).
4. Identify Spectator Ions:
Identify any ions that appear in the same form (with the same charge and same quantity) on both the reactant and product sides of the equation.
5. Eliminate Spectator Ions:
Cross out the spectator ions from both sides of the complete ionic equation.
6. Write the Net Ionic Equation:
Write the remaining ions and compounds as the net ionic equation, focusing on the chemical species directly involved in the reaction
Types of reactions
single-replacement reactions
A + BC → AC + B (or BA + C)
activity series
double-replacement reactions
AB + CD → AD + CB
neutralization reactions
acid + base → salt + water
solubility rules (SNAP)
SNAP = SOLUBLE = AQUEOUS
Sodium Ions (Na+)
Nitrate Ions (NO3-)
Ammonium Ions (NH4+)
Potassium Ions (K+)
synthesis reactions
Simple elements or compounds combine to create a more complex product
A + B → C
decomposition reactions
A chemical breaks down into at least 2 simple substances
A→ B + C
combustion reactions
a chemical reaction in which a fuel undergoes oxidation by reacting with an oxidizing agent, resulting in the release of energy
redox reactions
OILRIG
oxidation is loss, reduction is gain
Reducing agent = oxidized
Oxidation agent = reduced
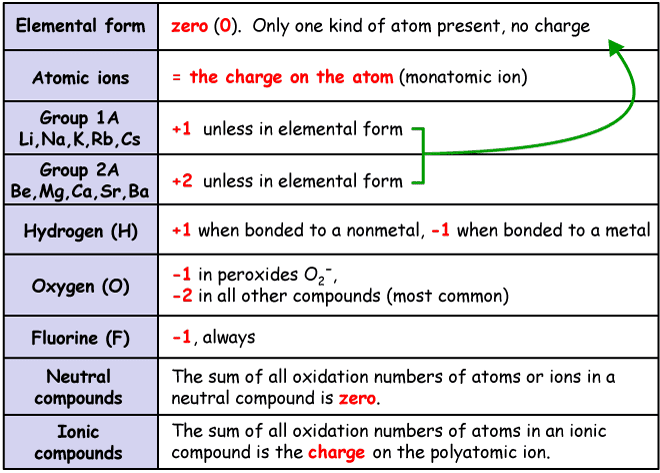
oxidation numbers
sign comes before number
pure element = 0
ions = charge
electron transfer
In a redox reaction, electrons are transferred from a substance (the reducing agent) to another substance (the oxidizing agent).
Oxidation:
Oxidation occurs when a substance loses electrons, which leads to an increase in its oxidation state.
Reduction:
Reduction occurs when a substance gains electrons, which results in a decrease in its oxidation state.
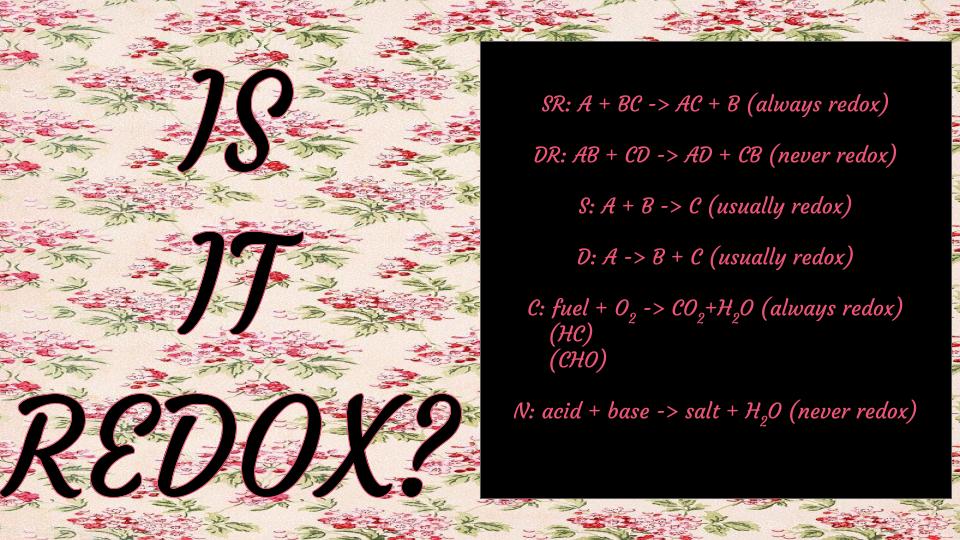
 Knowt
Knowt