What is solid:
What is solid:
- a solid as a material that retains its shape unless a compression force is applied
- Solid particle are made up of molecules that are held in close proximity to each other by intermolecular forces
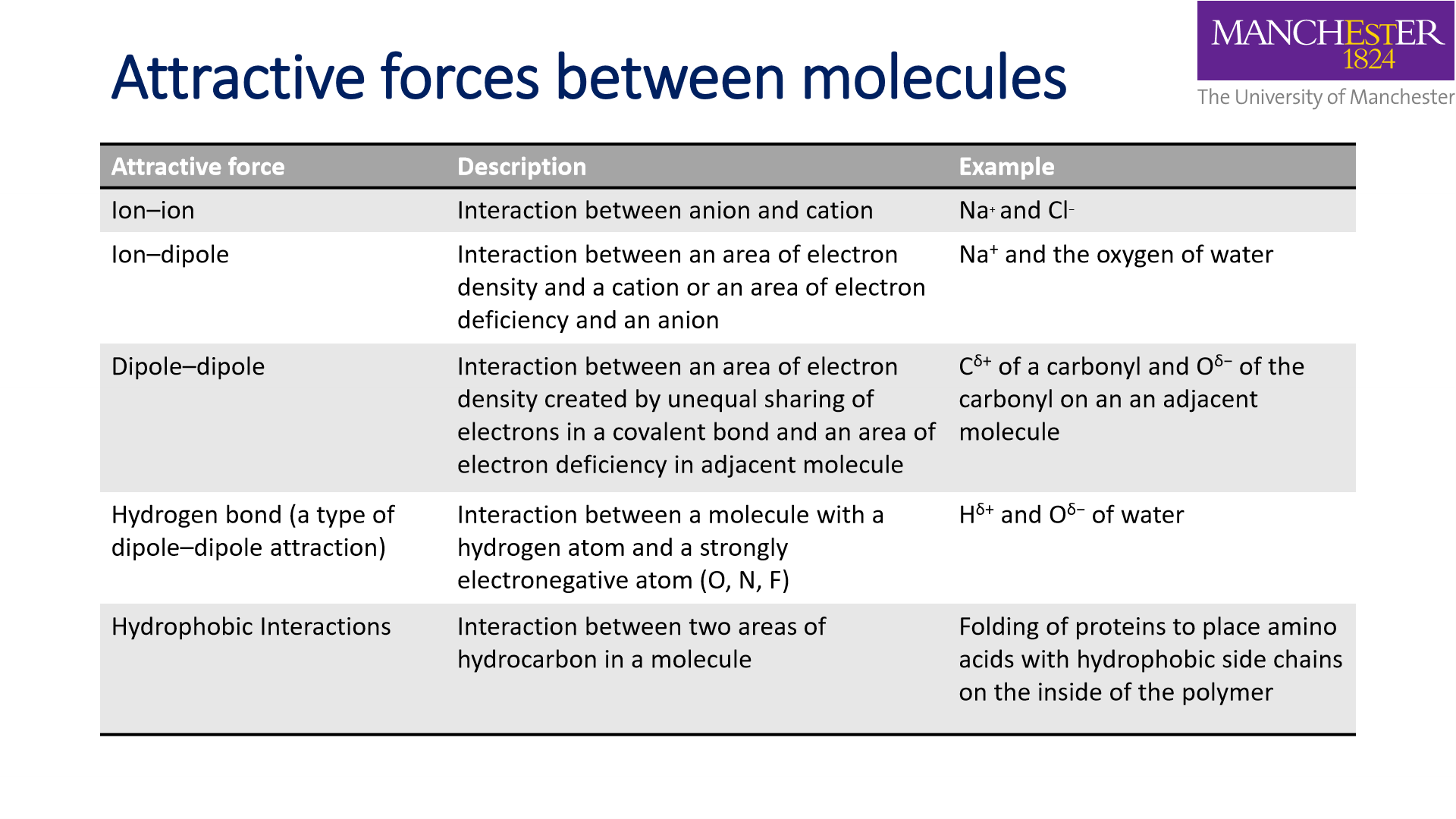
Attractive forces between molecules:
Characteristics of solids that affect the preparation and performance of solid dosage forms
- Arrangement of molecules
- Melting point
- Particle size, shape, surface energy
- Solubility
- Dissolution rate
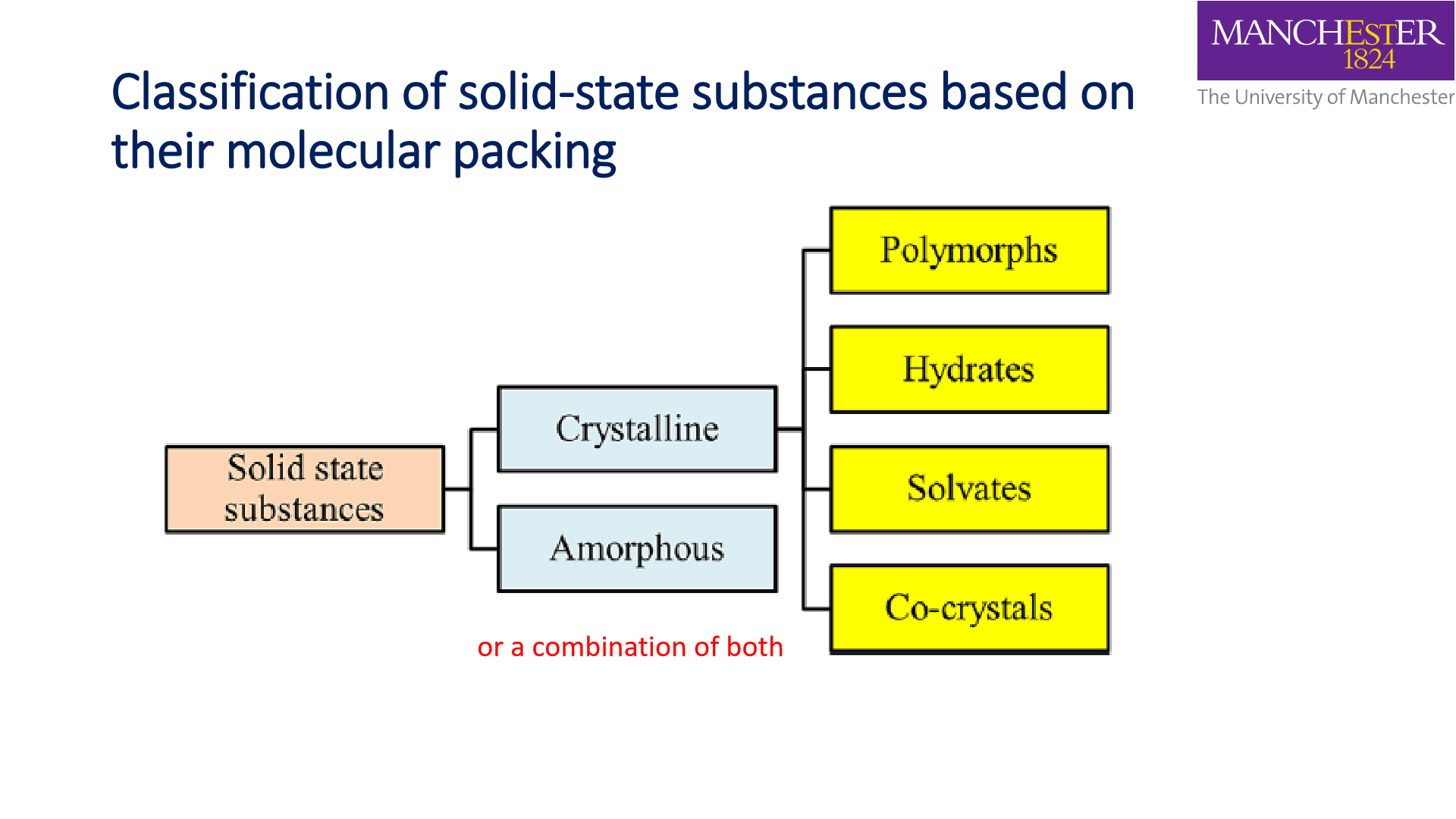
Polymorphs:
- Polymorphs are compounds that can exist in two or more crystalline structures; the chemical composition remains the same, but the arrangement of the molecules is changed.
- Packing polymorphism: same conformation of molecule but different packing
- Conformational polymorphism: different conformation of molecules
Polymorphism:
- Polymorphism influences:
- Melting point
- Solubility
- Dissolution rate
- Bioavailability
- E.g chloramphenicol palmitate has alpha and beta polymorphs, rate of absorption of beta polymorph is much high so have different bioavailability's and plasma levels
- Processing properties (powder flow + compressibility)
- Density
- Colour
- Polymorph with intermolecular attractive forces best aligned are more thermodynamically stable
- less stable polymorphs convert to more stable over time
Crystalline:
- Crystalline compounds exhibit a repeating structure (the unit cell), forming the crystal lattice.
Methods of crystallisation:
- Crystallisation is used to separate solute from solvents
- Evaporative crystallisation is a process in which a solution is concentrated by evaporating the solvent, leading to the precipitation of the solute as crystals. This technique is commonly used in various industries for the production of high-purity crystals or for separating and purifying substances from solutions.
- Cooling crystallisation is a process in which a solution is cooled to induce the crystallization of a solute. This method is commonly used to produce solid crystals from a solution by reducing the temperature, which decreases the solubility of the solute and promotes its precipitation in a crystalline form. Cooling crystallization is widely employed in various industries for the production of high-purity crystals, purification of substances, and isolation of specific compounds.
- Precipitation crystallisation is a process in which a solute is brought out of a solution to form solid crystals. This occurs when the solubility of the solute in the solvent is exceeded due to changes in conditions such as temperature, pressure, or the addition of a second solvent. The term "precipitation" in this context refers to the formation of solid particles (crystals) from a solution.
Control of crystallisation:
- Crystallisation depends on:
- Supersaturation state
- Nucleation after seeding
- Rate of cooling
Crystalline habit:
- Crystalline habit describes the shape of the drug crystal
- the shape of the drug particle is important to its flow dispensability and aerodynamic properties
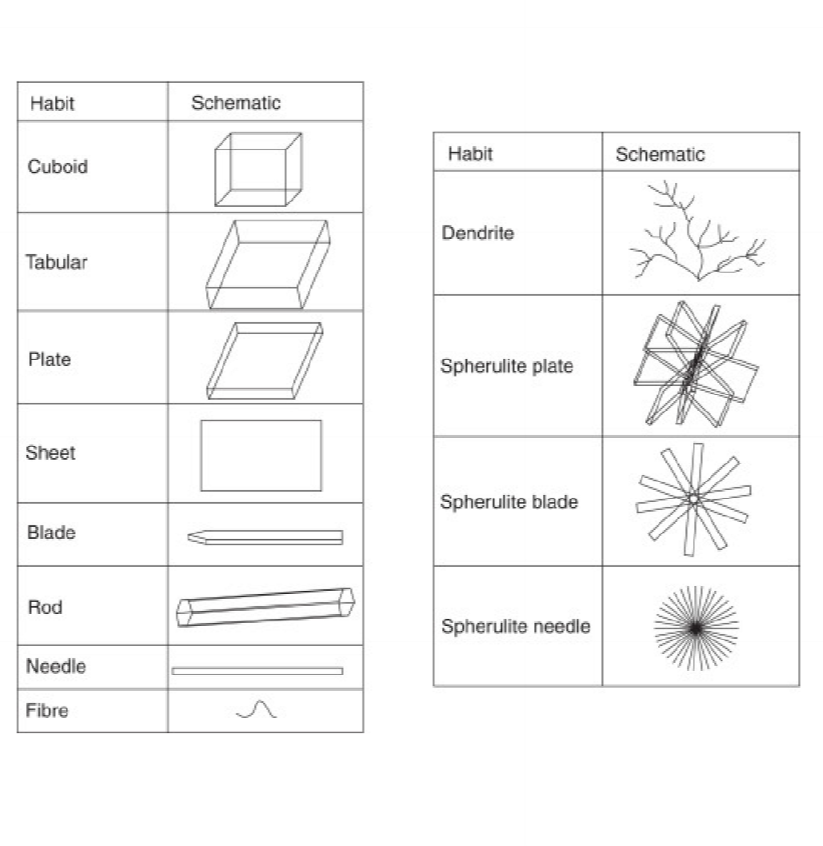
- Influenced by:
- Solvent
- Impurities
- Additives
- Physical conditions
Solvates:
- So they are orderly arrangements of drug molecules include solvent molecules in crystalline lattice
- if crystallisation solvent is water referred to as hydrates
- hydrates can lose water crystallisation by a process called efflorescence
- Hydrates may have different solubilities compared to anhydrous forms, as the water molecules can affect the interactions with solvents. - anhydrous forms are typically more rapidly soluble
- The colour of a hydrated compound might differ from its anhydrous form due to the presence of water molecules influencing electronic transitions.
- Some hydrates are stable only within a certain range of temperature and humidity. Anhydrous forms may be more stable under different conditions.
Co- crystals:
- Composed of an active drug species and Another organic molecule (co- former)
- Co- crystals can form between any two molecules independent of their state of matter.
- The co-former form hydrogen bonds with drug stoichiometrically providing a lower melting and more soluble solid than pure crystalline form
- Can be more stable than polymorphs of drug reducing concerns about polymorph instability in final dosage form
Hygroscopicity and deliquescence:
- hygroscopic powders take up water from the atmosphere
- drugs with a polar surface group will adsorb few molecular layers of water on the surface
- metal salts our prone too high hygroscopicity
- Deliquescent powders will adsorb water vapour from the environment and gradually form a solution - impacting by chemical stability
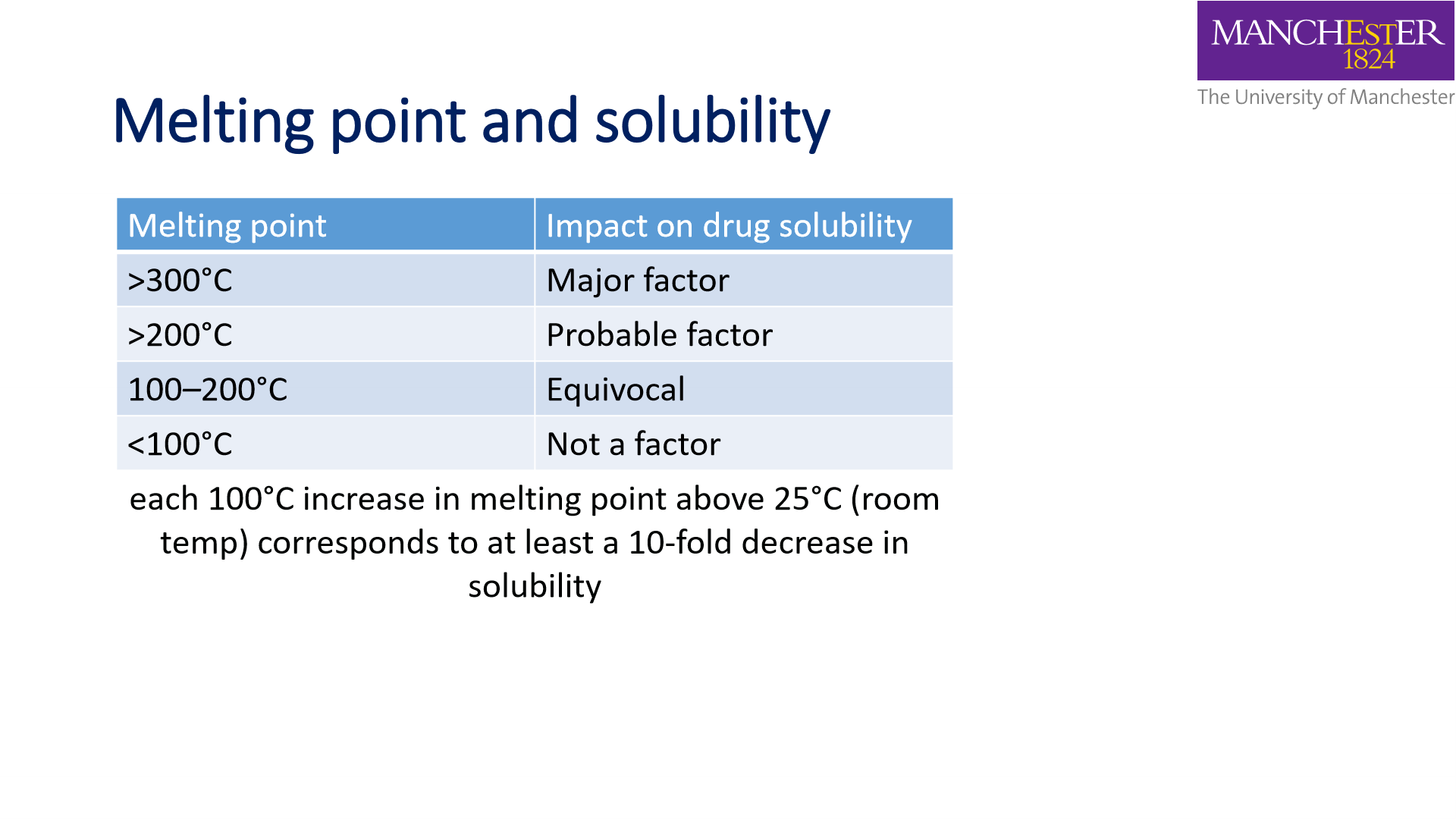
Melting point:
- Crystalline forms have a higher melting point than amorphous forms
- melting point measures strength of interactions between molecules
- high melting point:
- strong lattice
- Hard to remove
- low the solution rate
- low melting point:
- weak lattice
- easy to remove molecule
- high dissolution rate
Amorphous solids:
- No sharp melting point, mount over a range of temperatures
- no orderly arrangement of molecules within a solid
- can be formed when a drug solution is cooled rapidly that solute molecules lose mobility before assuming their lattice position
- Interactions between molecules in amorphous solids are easily disrupted so they have a lower melting point crystalline forms
- generally porous and low density
Solid dispersion and solid solution:

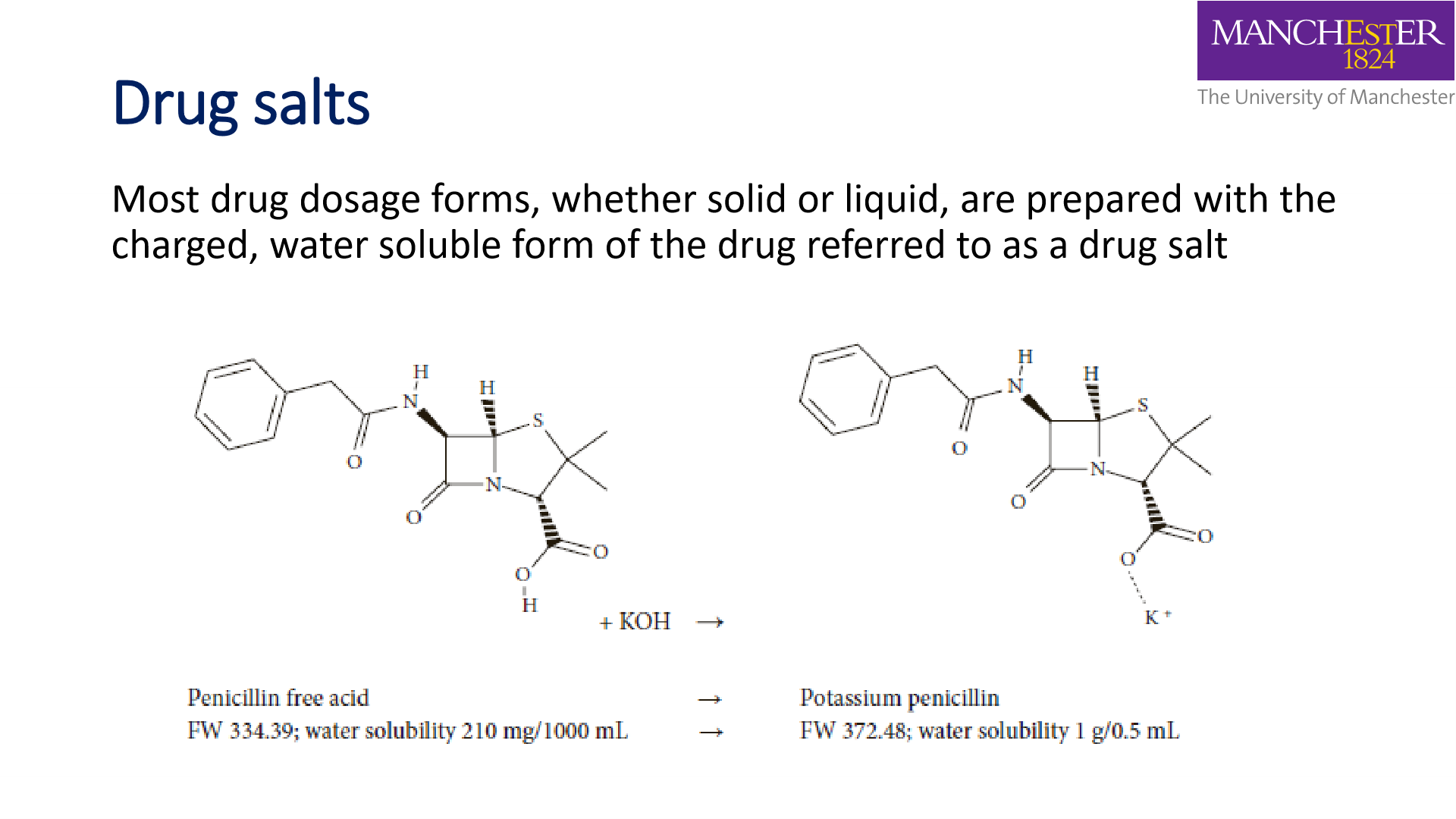
Drug salts
- Can be prepared from a reaction of weak base drug with a strong acid oh weak acid drug with a strong base
- A drug can have different salt forms
- physical properties can affect absorption thus soul forms are not regarded as pharmaceutically equivalent
Advantages of salt formulation | Disadvantages of salt formulation |
|---|---|
Enhanced solubility | Decrease percentage of active ingredient |
increase the solution rate | increased hygroscopicity |
higher melting point | decrease chemical stability |
lower hygroscopicity | increased number of polymorphs |
improved photo stability | reduce dissolution in gastric media |
better taste | no change in solubility in buffers |
higher bioavailability | corrosiveness |
better process ability | possible disproportionation |
easier synthesis or purification | additional manufacturing step |
potential for controlled release | increase toxicity |