Note
0.0(0)
AP Biology Study Guides
AP Biology Ultimate Guide
Unit 1: Chemistry of Life
Unit 2: Cell Structure and Function
Unit 3: Cellular Energetics
Unit 4: Cell Communication and Cell Cycle
Unit 5: Heredity
Unit 6: Gene Expression and Regulation
Unit 7: Natural Selection
Unit 8: Ecology
Studying for another AP Exam?
Check out our other AP study guides
Unit 1- Chemistry of LIfe
Module 0.1- Introduction
- Biology- the study of life
- Biologists- scientists that study life and explore the underlying principles and processes that shape and mold biological organism
- Organisms- living things/ beings that display all of the properties of life
- biology gives us an organized way of understanding ourselves, other living things, and the world
- Gene- the unit of heredity
- Genome- all the genetic info an organism contains
- help find ways to fight disease in people, animals, and plants
- endangered species and evolution
- Cells- the simplest self-reproducing unit that can exist independently
- Biology helps us learn world processes, investigate life, ask scientific questions, and make informed bio decisions
- 4 big biology ideas- evolution, energetics, info storage+transmissions, and system interactions
Evolution
- Species- a group of interbreeding organisms that produce fertile offspring, usually distinct by form, behavior, or biochemical properties
- Unity and diversity of life- a distinct trait that differentiates types but similarities can explain similarities
- Evolution- change over time, explains unity and diversity of life
- the concept that unites all of biology
- the key principle of life
- Unity and diversity occurs in the smallest single-celled organisms to the largest animal ever
- Natural selection- described by Charles Darwin and Alfred Russel Wallace, mechanism of evolution in which some individuals survive and produce more than others in the environment, passing variation
- how crops are developed, breeds of animals, why bacteria stop responding to antibiotics
- shapes predator and prey interactions
- use skills from the nervous, sensory, musculoskeletal, endocrine, circulatory, and respiratory systems
- Artificial selection- humans decide the best traits, decide which trait reproduces
- Competition- fighting for resources, natural selection, the winner continues
Energetics
- Energy- ability to work, necessary for life, needed to survive, grow, move, and reproduce
- Energetics- examines properties of energy, how energy is distributed in bio, chemistry, and physical processes
- use of energy is a unifying characteristic of species
Information Storage and Transmission
- Information- instructions that all cells have that in part determine what they look like and the function
- DNA- where cell information is stored, carrier of all genetic information
- need access to grow and carry out functions
- guides the development of offspring
- variations allow some organisms to survive in and reproduce in different environments
System interactions
- System- a group of things that function together as a whole
- can be small or large and involve necessary nonliving materials
- Biotic- living organisms
- Abiotic- nonliving components
- Biological system- biotic and abiotic entities that interact, and have complex properties
- simple to complex interactions, action among system lead to new emergent properties
- Emergent property- a property of a system that the individual parts don’t have on their own
- when interacting components are viewed in an organism that is an emergent property or LIFE
- diversity and complexity of biosystems enable robustness to help tolerate and respond to environmental changes
- Robustness- ability to tolerate and respond to environmental change
Module 0.2
Scientific inquiry is a deliberate way of asking and answering questions about nature
- Scientific inquiry- process scientists use to ask questions and seek answers about the natural world in a deliberate and ordered way
- limited to investigations of the natural world
- provides opportunities to observe, investigate, and explain how natural phenomena occur
- consists of exploration, investigation, and communications
Making observations and asking questions
- Exploration phase- the first step of scientific inquiry, make observations and ask questions
- Observation- the act of viewing the world around us, allows us to make focused questions about nature
- used to refine questions
- good questions are the fundamental component of thinking like a scientist
Formulating Hypotheses
- observation and critical thinking helps propose a hypothesis
- Hypothesis- tentative explanation for one or more observations, make predictions that can be tested by experimentation or additional observations
- the working explanation that helps a researcher understand observation and leads to a better understanding
- lead to further observations or experimentation
- test hypothesis, determine if predictions are supported
- data from testing or observation, analyze data to see if it supports or rejects the hypothesis
- doesn’t prove a hypothesis, just that isn’t false
Designing controlled experiments
- Controlled experiment- now of the most powerful types of experiments, at least two groups tested, identical test and set up but with one single variable change
- Test group/ experimental group- group experiencing the change/ variable
- Control group- not exposed to a variable
- determine if the variable accounts for any change
- Independent variable- variable manipulated to test the hypothesis, “independent” can be manipulated
- Dependent variable- the result of the experiment, “dependent” vary based on the independent variable
- test to see if that variable is important
- Negative control group- control group, expect to see no effect, test again that independent variable causes the change
- Positive control group- receives a variable with a known trait
- when experiments and observations don’t support the hypothesis they modify or reject it
- when experiments and observation do support the hypothesis it is subject to more tests and observations
- only supported but not proven
Analyzing and interpreting data
- data is the bedrock of science
- data is observations, measurements, and facts
- either qualitative or quantitative
- Qualitative- descriptive
- Quantitative- number form, statistical analysis
- Statistical analysis- interpret the collected data
- averages, spreads, and reals from groups
- Null hypothesis- predicts that an invention or treatment has no effect, chance, and not the variable
- not a real difference between the controlled and independent variable
- Alternate hypothesis- predicts that the invention or treatment has an effect
- the difference between the control and independent variable is real
- P-value- expresses the likelihood that an observation was by chance, a probability
- a P-value less than or equal to 0.05/ 5% means that there is equal to or less than a 5% chance an observation was seen by chance and rejects the null hypothesis
- a P-value greater than 0.05/ 5% means that the observation was most likely taken by chance and fails to reject the null hypothesis
- Error bar- a short vertical line showing a range of values, graphically shows uncertainty, shows a range of values that incorporate small differences among individuals and inaccuracies
Communicating findings
- a critical step in scientific inquiry and guides research and public action
- communicated through journals, meetings, and conferences
- informs the public and other scientists leading to more questions
- goes in a circle of questions to experiments to more questions
- good scientists learn from failed experiments and plan new ways of approaching problems
Establishing Theories
- a hypothesis may originally be tentative and provide one or several possible ways to explain an observation
- with repeated observation and experimentation a good hypothesis gathers strength and researchers become more confident
- Theory- multiple related hypotheses survive repeated testing and become accepted as a good basis for explaining what is seen in nature; a general explanation of the world supported by a large body of experimental evidence and observation
- examples: gravity theory, chromosome theory, germ theory, cell theory, and the theory of evolution
- in general conversation, the word theory is usually used as a “hypothesis” or “idea”
- in science, a hypothesis is only a theory if it withstood testing and has a general explanation for many results
- a good theory generates hypotheses and predicts the outcomes
- evolution is one of the most significant theories in biology
- a general and powerful explanation of how life works
Statistics tutorial
Significant Figures
- Appropriate precision/ significant figures- indicate the precision of a measurement
- the more significant figures the more precise
- all non-zeros are considered significant, zeros between non-zeros, and zeros after decimal points after non-zeros
- significant figures in your final answer should be the same as the least precise data piece
Average: mean, median, and mode
- Average- individual pieces of data represented as one single number
Mean
- Mean- most common average, add up all values in the data set and divide by the number of data pieces in the data set
Median
- Median- middle value in a group of values
- less influenced by the extremes than the mean
- Outlier- a data point that is very different from the others
- list the values in numerical order and identify the value in the middle of the list
Mode
- the value that shows up the most
Spread of data
- Distribution- the spread of all numbers in experimental results
- Normal distribution- natural phenomena graph, smooth, bell-shaped curve
- the peak is the mode
- an equal number on both sides
- the middle is the median
Range
- Range- a way to quantify how spread out the data is, the highest value mimics the lowest
- highly influenced by outliers
Standard deviation
- Standard deviation (S)- shows spread, how far data in the data set are from the mean
- smaller standard deviation means the data points are closer to the mean
- influenced by outsiders/ outliers but not as much as the range
- 68% of values fall on either side of the mean and 95% in two standard deviations
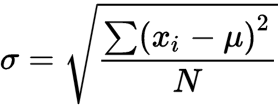
Percent change
- change in percent
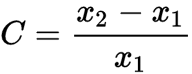
Error
- Human error- how human error was avoided
- Calibration/ instrument error- how it was avoided, calibrate and check instruments
- Material error- defective material, should be consistent
- Measurement error- error in measurement, the more measurements the more room for error
- Uncontrolled variable- random things like humidity, leads to further questions
Symbols
- N- population size
- n- sample size
- x̅- mean
- S- standard deviation, variation in sample
- xi- data points
- Σ- semation
- N – 1- degrees of freedom
- SEX- confidence or precision of a measurement or answer
Module 1- Elements of life
Matter and energy govern the properties of life
- Matter- anything that has mass and takes up space, material that makes up physical objects
- gas, liquid, or solid
- all organisms exchange matter with their environment- grow, reproduce, maintain, and maintain organization
- Atom- the basic unit of matter
- Molecules- chemicals made up of two or more atoms
Flow of Energy
- An energy source for many organisms is carbon-rich organic molecules
- energy doesn’t move in a cycle and rather must be harvested from the environment
- the sun is usually the entry point for energy into living systems
- plants, algae, and certain bacteria capture energy from the sun and synthesize it
- other organisms then eat those plants
- when the sun is absent, energy comes from chemical compounds
- organisms transfer energy or acquire energy from the environment and convert it into a chemical form that their cell can use
- extra energy is dissipated as heat if not used to build cellular components, move, or reproduce
Module 1.1- The atom is the fundamental unit of matter
- 2 million species exist and 10-100 million have ever existed
- diversity is seen in the molecules of a cell
- they change critical characteristics
- a couple of organic molecules make up the few types of atoms
Atomic structure
- Nucleus- the dense center of an atom
- Protons- positively charged particles in the nucleus, determine the element
- Neutrons- electrically neutral particles in the nucleus, determine the isotope and how radioactive it is
- Electrons- negatively charged particles in the nucleus, determine its reactivity
- Atomic number- number of protons, subscript
- Element- a chemical that can’t be further broken down, specified by the atomic number
- the proton and neutron have an atomic mass of 1, electron weight is negligible
- Atomic mass- the total mass of an atom, number of protons and neutrons, subscript to the left of the symbol
- the number of neutrons can differ and change its mass
- Isotopes- atoms of the same element with different numbers of neutrons
- an atom typically has the same number of protons and electrons
- a chemical process can cause an atom to gain or lose an electron
- a lost electron becomes a cation
- and a gained electron becomes an anion
Electrons
- the foundation of energy transfer in many bio reactions is the movement of electrons
- electrons move around the nucleus in a cloud, the denser the area the more likely the electron is there
- Energy level or electron shell- area in the space where the electron circles the nucleus
- many important bio atoms have two energy levels, the second level can hold up to eight electrons
- electrons closer to the nucleus have less energy and are less reactive
- atoms are most stable when the energy level is full
- most stable when the closest levels fill first as well
- molecules are often formed by sharing electrons
- if an electron is lost a hydrogen ion forms because it has one more proton than electron
Chemical properties of elements
- Valence electrons- electrons in the outermost energy level
- Periodic table of elements- describes valence electrons and their properties
- arranged by the atomic number
- elements in the same row have the same energy levels, across the row elements to the right have one more electrons
- the last element in each row has a full complement of electrons
- family/ group- vertical column, the same number of electrons in the outermost ring/ level
- determine how elements interact to form a diversity of molecules
- the four elements common to all organisms are carbon, hydrogen, oxygen, and nitrogen
- organisms use elements in the first five rows of the table
- the most used are rows one and two and phosphorus
Module 1.3 Notes - Atoms combine to form molecules linked by chemical bonds
- diversity in life is determined by atom combinations
- Molecules- groups of 2 or more atoms bonded together to act as a single unit
- Chemical bond- how individual atoms interact, type of attraction between atoms that hold them together
Covalent bonds
- Valence electrons- electrons furthest from the nucleus, determine the ability of the atom to combine
- when atoms combine they share valence electrons, and the shells overlap
- Covalent bonds- a pair of electrons are shared between atoms, non-metals
- are most stable when they share electrons to fill an energy level
- the lowest possible energy state requires significant energy input
- Single bond- two atoms share two electrons in a covalent bond
- Double bond- two atoms share two pairs of electrons covalently, shown by a = connection
Polar covalent bonds
- electrons aren’t usually equally shared
- caused by a difference in the ability of the atoms to attract electrons
- Electronegativity- an atom’s ability to attract electrons
- Polar covalent bonds- electrons are shared unequally between two atoms
- Nonpolar covalent bonds- a covalent bond where electrons are shared equally
- atoms with similar electronegativities are usually non-polar
- electronegativity tens to increase across a row on the periodic table
- the number of positively charged protons across a row increases, the negative electrons are held more closely
Ionic bonds
- Ion- bond where one atom is electronegative and it takes an electron that is less electronegative
- the atom that took an electron now has a negative charge
- the atom that lost an electron now has a positive charge
- shown by superscript charges
- chemicals that dissolve in water tend to have polar or charged regions
Chemical Reactions
- chemical bonds that link atoms in molecules can change in a chemical reaction
- Chemical reaction- a process where atoms or molecules are transformed into different molecules
- Reactants- atoms or molecules that are changed in a chemical reaction
- Products- molecules formed from a reaction
- chemical reactions provide a way to build and break down molecules for use by the cell, as well as to harness the energy that is held in chemical bonds
Module 1.4 - Carbon is the backbone of organic molecules
The chemistry of carbon
- hydrogen and helium are the most abundant elements in the universe
- Earth is mostly silicon, oxygen, aluminum, iron, and calcium
- human cells are mostly carbon, oxygen, hydrogen, then nitrogen
- most other cells have the same ratios
- Organic molecules- carbon-containing molecules
- make up the structure of cells. participate in and speed up reactions, and store energy
- 4 of the electrons in a carbon atom are in the outermost shell and available to form a tetrahedron
- forms 4 covalent hydrogen bonds
- because these can move freely carbon can make compounds in a variety of shapes
- can perform a variety of necessary functions
- carbon atoms can covalently bond and form long chains
- either branched or 2 carbons in the chain that can form a ring
- carbon’s ability to take many forms is why it is so good for life
- silicon can act semi-similar to carbon but will usually bond with oxygen
Organic Molecules
- there are 4 classes of organic molecules:
- all are carbon-containing
- proteins
- nucleic acids
- carbohydrates
- lipids
- Monomers- repeating subunits, come together to make polymers
- Polymers- long chains, built from monomers
- Proteins- organic molecules, do much of the cell’s work
- can speed up chemical reactions and provide structural support to the cell
- Amino acids- the subunits that make up proteins
- each cell has 1000s of proteins with all different functions that are dependent on the amino acids
- Nucleic acids- encode and transmit genetic information
- deoxyribonucleic acid (DNA)- genetic material, transmitted from the parent to the offspring
- ribonucleic acid (RNA)- synthesizes proteins
- Nucleotides- repeating subunits that make up nucleic acids
- Nucleic acids are information molecules that carry info in the sequence of nucleotides
- Carbohydrates- sugars, organic molecules that store sugar in their chemical bonds
- sometimes attach to proteins on cell surfaces
- glucose, galactose, lactose
- composed of monosaccharides
- Lipids- hydrophobic organic molecules
- associate more with other lipids
- chemically and functionally diverse
- effective membranes and barriers between cell interiors and the environment
- makeup signaling molecules and energy-storing fats
- Hydrophobic- “water-fearing”, non-polar molecules that don’t dissolve in water
- Hydrophilic- “water-loving”, polar molecules that readily dissolve in water
Module 2
Module 2.1- Life depends on the properties of water
- all life on earth depends on water
- where life originated
- influences habitats
- water is the most abundant molecule in cells
- due to its polar covalent bonds
- Polar molecules- regions of positive and negative charges
Hydrogen bonds
- Hydrogen bond- hydrogen atom interacting with an electronegative atom (opposite charge)
- much weaker than covalent and ionic bonds
- can be strong when multiple of them
- help stabilize organic molecules like nucleic acids and proteins
Cohesion, adhesion, and surface tension
- Cohesion- molecules stick to one another
- happens in water molecules
- Adhesion- the tendency for molecules to stick to other things
- happens with water
- Surface tension- the measure of the difficulty of breaking the surface of a liquid
- a consequence of cohesion
- happens because surface molecules don’t have other molecules to stick to
- allows things to float on water
The structure of water
- hydrogen atoms account for the form of water
- ice- hydrogen and 4 other water molecules, form an open crystalized lattice, less dense
- water- hydrogen bonds destabilize and break, denser, more hydrogen bonds
- steam- water molecules are too far apart to make hydrogen bonds, too much heat and energy, and no crystalline structure
Specific heat
- more hydrogen bonds make changing the form of water more difficult
- heat breaks apart hydrogen bonds and the energy isn’t able to make the water molecules move more
- Specific heat- the amount of energy to change the heat of a substance
- heat/ unit of mass
- water’s specific heat is 1 cal/ 1 gram
- good because it resists heat changes in cells from chemical reactions
- water habitats change temperature less and therefore are more stable
- the sea and ocean temperature help to regulate the earth's temperature
Module 2.2- Water is the Medium of Life
Solvent properties of water
- hydrophilic molecules dissolve in water and therefore fo through hydrogen bonding
- Solvent- capable of dissolving many substances
- many organic molecules dissolve in the solvent, water
- hydrophobic molecules, typically nonpolar, don’t dissolve in water
- important for processes like protein folding
Water dissociation and pH
- water doesn’t always exist as an intact molecule- 1 oxygen and one hydrogen
- can become an H+ and an OH- molecule (hydroxide ion)
- oxygen takes an election from one of the hydrogens
- can affect the function of proteins, nucleic acids, and carbohydrates
- affects how basic or acidic
- Acids- donate H+ ions to solutions, acidic
- Bases- molecules that accept H+ ions or remove them from solutions, alkaline
- Neutral- the concentration of H+ and OH- ions is equal
- pH scale- a way of describing how acidic or basic a solution is, the concentration of H+
- 7 is neutral
- less than 7 is acidic
- more than 7 is basic
- pH=-log[H+]
- a small pH change is a large change in H+ concentration
Module 2.3- Dehydration synthesis reactions build molecules, and hydrolysis reactions break them down
Dehydration Synthesis reactions
- in some chemical reactions, a water molecule is released as the covalent bond is formed
- Dehydration synthesis- water is removed during the chemical reaction, and larger molecules become smaller
- helpful in making cells for cellular processes
- sometimes add a unit to a molecules chain, organic molecules
Hydrolysis reaction
- break down molecules for energy, or to be taken up and used, how we break down food
- Hydrolysis reaction- chemical reactions that break covalent bonds by adding water
- one product gets an H and another gets and OH
Water
- polar
- cohesive
- adhesive
- high surface tension
- less dense as a solid than a liquid
- high specific heat
- good solvent
- reactive
Module 3- Carbohydrate and Lipids
- carbohydrates and lipids are energy-rich molecules that fuel other molecules
- have many carbon-carbon and carbon-hydrogen bonds
- carbohydrates are stored as glycogen and can provide long-term energy
- also can make up cell walls
- lipids form barriers between the inside and outside of a cell
- some are chemical messengers and some send messages between cells
Modules 3.1- Monosaccharides are the basic units of carbohydrates
- carbohydrates are also known as sugars or saccharides
- carbohydrates can be cell energy reserves
- carbohydrates can form rigid structures in the cell wall
- monosaccharides are the carbohydrate subunits or single sugars
Monosaccharides
- simple carbohydrates/ saccharides that contain 5 or 6 carbons- C6H12O6
- different have different arrangments of the atoms
- isomers- molecules that have the same chemical formula but differ in structure
- many monosaccharides have CH2O which is the same ratio as saccharides
- Functional group- groups of one or more atoms that have particular chemical properties, regardless of what they are attached to
- Ex. hydroxyl group[ (-OH) in glucose and fructose, polar, and hydrophilic
- Ex. carbonyl group, polar, hydrophilic, in glucose and fructose
Linear and ring structures
- linear- form a straight chain of carbon atoms, glucose and fructose
- most monosaccharides form ring structures where the ring folds on itself
- once the ring is formed carbon atoms go above and below the ring
- the ring shape affects the function of the molecules
- 6 carbon sugars are among the smallest carbohydrates
- Ribose- 5 carbon sugar, a component of nucleic acid RNA
- Deoxyribose- 5 carbon sugar, a component of nucleic acid DNA
- some carbohydrates only have carbon, hydrogen, and oxygen, some also have nitrogen and phosphorus
Module 3.2- Monosaccharides are joined by glycosidic bonds to make complex carbohydrates
- monosaccharides, especially 6-carbon sugars make long chains or branching molecules
- the function of carbohydrates depends on their shape and arrangement of monosaccharides
- Disaccharide- two simple sugars linked together by a covalent bond
- Polysaccharides- a type of polymer, depend on how simple sugars combine
- Complex carbohydrates- long-branched chains of monosaccharides
- glycosidic bonds- covalent bonds that attach to monosaccharides
- starch- energy storage in a molecules
- cellulose- tough, resilient molecules, give strength to plant cell walls and stems
- the most widespread organic molecule on earth
Module 3.3- Lipids are hydrophobic molecules
- lipids are defined by a particular chemical composition
- defined by being hydrophobic
- organisms require lipids
- store energy
- cell communication
- Cell membranes/ plasma membranes- structures that define boundaries between the inside and outside of a cell, lipids are a major component
Triclyglycerols
- Triglycerol- lipid that’s used for energy storage, a major component of animal fat and vegetable oil
- made up of glycerol joined to three fatty acids
- can contain different fatty acids attached to the glycerol backbone
- Glycerol- 3 carbon molecules with hydroxyl (-OH) groups attached to each carbon
- Fatty acids- long chain of carbon atoms, called a hydrogen carbon chain, attached to a carbonyl group, no polar bonds
- can react with a hydroxyl group of a glycerol atom
- differ in tail length/ the number of carbon atoms in the hydrocarbon chain
- longer tail more energy
- differ in the number of carbon-carbon double bonds
- can be packed into a small area
- dehydration synthesis reactions link chains of sugar molecules
- Saturated- fatty acids that don’t contain double bonds
- carbon connected to 2 hydrogens
- has a higher melting point
- straight chains
- Unsaturated- fatty acids that contain carbon-carbon double bonds
- at least 2 carbon atoms aren’t attached to 2 hydrogen atoms
- lower melting point
- kink or bend at each double bond
- Van der Waals forces- the interaction of temporary molecules because of opposite forces
- fatty acids have these
- weaker than hydrogen bonds and need to be very close together
- changes melting point
- longer hydrogen means a higher melting point
- kinks from double bonds reduce the tightness and therefore lower the melting point
Steroids and phospholipids
- Steroids- a type of lipid, 20 carbon atoms bonded to form 4 fused rings, an example is hormones
- cholesterol- a type of steroid, starting point for animals to make other steroids, 20 carbons that make 4 rings
- many carbon-carbon and carbon-hydrogen bonds
- very hydrophobic
- Phospholipids- a type of lipid, a major part of cell membranes, glycerol backbone
- glycerol backbone attached to a fatty acid, attached to 2 fatty acids
- called the phospholipid tails, hydrophobic
- glycerol attached to a chemical structure which includes a phosphate group
- the phosphorus forms the phospholipid head
- the phosphate group is a hydrophobic functional group
- 2 layers of phospholipids make up the cell membrane
Module 4- Proteins
Module 4.1- Amino acids are the building blocks of proteins
- proteins help create the cell’s structure
- amino acids- subunits that makeup proteins
- change protein function
- primary organic molecules in the function of the cell
Amino acid structure
- the amino acid structure is an alpha carbon, connected to 4 chemical groups
- amine group- (NH2)
- Carboxyl group (COOH)
- a hydrogen atom (H)
- R group
- R-group- differs from one amino acid to the next, what makes amino acids different, changes their chemical and physical properties
- in a normal cell environment (pH of 7.4) the amino and carboxyl groups are ionized or charged
- the amine group gains a proton
- carboxyl group loses a proton
Twenty common amino acids
- 20 different amino acids make up proteins
- hydrophobic amino acids have nonpolar amino acids
- aggregate with each other
- hydrophilic amino acids are either polar, basic, or acidic
- basic amino acids gain a proton and acidic ones lose one
- R-groups have one slightly more negative end
- charged amino acids are on the outside because they associate with other molecules and proteins
- glycine, proline, and cysteine have special properties
- glycine- the R-group is a hydrogen atom, nonpolar, and can freely move
- increases flexibility
- proline- has a carbon chain that puts kinks in the structure of proteins
- cysteine- affects folding with its sulfhydryl (-SH) group
- can form S-S disulfide bonds
Functional groups
- Functional group- one or more atoms that have particular chemical properties no matter what they’re attached to
- many R groups also contain functional groups which give amino acids their properties
- many functional groups are reactive and polar
- nonpolar molecules become polar and soluble when they contain these groups
Functions
- metabolize enzymes- control reactions in cells, specific to a reactor and certain conditions
- structural proteins- structural stability and allow for movement
- contractile proteins- actin and myosin allow muscles to move and contract, move intercellular components
- transport proteins- proteins in a cell’s membrane which control the movement of substances in and out of the cell
- hemoglobin- transports substances through the blood to all parts of the body
- defense proteins- form antibodies that detect foreign substances in our bodies and attack them
- hormonal regulation and communication proteins- serve as intracellular messengers that influence the actions of cells
- receptor proteins- receive and respond to molecular signals to control or regulate processes
- genetic regulation proteins- transcription factors that control genetic expression
Module 4.2- Peptide bonds link successive amino acids to form proteins
- the order of amino acids changes how a protein folds
Peptide bonds
- the carbon atom in a carboxyl group of an amino acid is joined to the nitrogen atom in the amine group of the next
- Peptide bond- covalently linked amino acids
- connected through dehydration synthesis reactions, the carboxyl releases OH, and the amine releases H
- results in 2 amino acids
- carbonyl/ carboxyl group (c terminus)- one end of the amino acid chain
- amine group (n terminus)- another end of the amino acid chain
- Peptide- short polymer of amino acids
- Polypeptides- a long polymer of amino acids, typically proteins
Amino acid sequence
- Primary structure- sequence, or order of amino acids in a protein
- can give proteins positive and negative areas
- usually represented by a series of 3 or 1-letter abbreviations
The environment can change protein structure and function
- an increased temperature increases molecular movement and can break weak bonds like hydrogen bonds
- change the protein structure
- pH concentration- changes the ionization of side groups and can expose the carboxyl and amine groups
- can change ion attraction and repulsion
- polar substances- can disrupt hydrogen bonding
- ion polar substances- can block or disrupt hydrophobic groups
- denaturing- a change to a protein that causes it to lose its functions, many times a reversible change
Module 4.3- Proteins are folded into 3D shapes that determine their functions
Secondary structures
- Secondary structures- formed through interactions between stretches of amino acids
- hydrogen bonds occur between different polypeptides allowing for more folds
- types of secondary structures
- alpha helix- polypeptide backbone is twisted forming a spiral or helix
- stabilized by hydrogen bonds that form between each carboxyl group and amine group
- Beta-sheet- polypeptide forms a pleated sheet that is stabilized by its hydrogen bonds between carboxyl groups in one chain and an amine group in another chain
- usually shown with broad arrows
- antiparallel- the arrows run in opposite directions
Tertiary structure
- Tertiary structure- the 3D shape of a single polypeptide chain
- defined by interactions between amino acid R groups
- special distribution of hydrophobic R groups, either ionic or hydrogen bonds
- usually loops or turns in the backbone that allows R groups to be close and bond
- determined by the primary structure
- function and the tertiary structure are affected by the contours and distribution of charges and have pockets that might bind with smaller molecules on the inside which allows proteins to be structural support, in membrane channels, and signaling molecules
- proteins with incorrect amino acids can’t fold properly and can become inactive
Quarternary structure
- Quarternary structure- proteins with two or more polypeptide chains
- their function is based on the tertiary structures that make them up
- polypeptides in the structure can be the same or different
- often have emergent properties that subunits don’t have on their own
- the sum is greater than its parts
Module 5- nucleic acids
Module 5.1- Nucleotides are the building blocks of nucleic acids
- DNA function- stores genetic information, encodes proteins, transmits genetic information
- nucleotides- monomers that make up DNA and RNA
structure of a nucleotide:
Nitrogen base- a main component of a nucleotide, a cyclic molecule that contains nitrogen, carbon, hydrogen, and oxygen
- Purines- double ring structures, adenine (A) and guanine (G)
- Pyrimidines- single-ring structures, thymine (T) and cytosine(C)
- the sequence and base change the nucleotide function
a 5-carbon sugar attached to each base which is indicated by a pentagon
- 4 of the 5 points represent the position of a carbon atom
- the sugar is deoxyribose, and the carbons in the sugars are labeled with a ‘
- in deoxyribose, the 2’ carbon has a hydrogen atom and the 3’ has a hydroxyl group
a phosphate group
a central phosphorus atom covalently bonded to 4 oxygen atoms
has a polar and negatively charged functional group
attaches to the 5’ carbon atom, negative charges on 2 of its oxygen atoms
- the free hydroxyl groups attached to the phosphoric atom are ionized by the proton loss
- negative charges make DNA a mild acid, they lose protons to an aqueous environment
Module 5.2- Phosphodiester bonds join nucleotides to form nucleic acids
- DNA- large molecules, consisting of many nucleotides
- phosphodiester bonds- bond nucleotides, a series of bonds
- can withstand heat and substantial changes in pH
- DNA backbone- 5 carbon sugar and a phosphate group
- each sugar linked to the neighboring phosphate group
- Directionality/ polarity- caused by phosphodiester linkages, one end of the DNA differs from the other
- 5’ end- nucleotide at the top, has a free 5’ phosphate, and one end of the nucleotide
- 3’ end- nucleotide at the bottom, has a free 3’ hydroxyl, other end
- DNA and RNA can polymerize (grow)through new phosphodiester bonds
- when a nucleotide triphosphate, joins a growing chain- DNA synthesis
- reacts with existing DNA molecules, connects 3’ end
- only 1 of the 3 phosphate groups from the joining nucleotide triphosphate is used to make the sugar-phosphate backbone
- other 2 released as pyrophosphate or 2 phosphate groups (attached)
Module 5.3- Cellular DNA takes the form of a double helix
- Double helix- DNA has 2 chains wound together, each strong in a helix
Double helix
- repeating structure
- number of A and T is always equal and the number of G and C is always equal
- backbone pointing outward, bases inward, A and T paired and G and C paired
- how DNA is stored, replicated, and direct synthesis of other macromolecules
- DNA strands are antiparallel- run in opposite directions, the 3’ end and 5’ end are opposite
- strands go in opposite directions
Base pairing
- each base pair contains a purine and pyrimidines
- between to purines would cause a bulge
- pyrimidines would cause a narrowing
- Complementary- form specific pairs
- knowing the base sequence in one chain tells you the other
- A and T form 2 hydrogen bonds
- O or N shares a K with another electronegative atom across the strand
- G and C form 3 hydrogen bonds
- O or N shares a K with another electronegative atom across the strand
Module 5.4- DNA and RNA have similarities and differences
- DNA and RNA are nucleic acids so they have similarities
- polymers
- directionality/ polarity- 5’ phosphate group and 3’ hydroxyl group
- DNA sugar is deoxyribose and RNA sugar is ribose- 2’ has a hydroxyl
- hydroxyl groups are reactive which makes RNA less stable
- RNA has 4 bases- 2 purines and 2 pyrimidines
- thymine is replaced with uracil
- U and A pair
- RNA molecules are usually much shorter with 1000s to millions of nucleotides
- Most RNA is single-stranded rather than a double helix
- can fold to form a 3D structure and create a double strand with itself
- DNA stores genetic information and transmits it which specifies the protein amino acids
- tells the function, chemical properties, and biological activities
- RNA function- intermediate between DNA and protein during protein synthesis
- Central dogma- the flow of info from DNA to RNA proteins
- DNA replication- all DNA is completely copied and transferred
- Transcription- DNA-RNA only portions of the DNA are transcribed
- Translation- RNA-Protein, expression of a gene
- mRNA- messenger RNA
- tRNA- transfer RNA
- rRNA- ribosomal RNA
- coenzymes- act as enzymes but aren’t proteins
- ATP to ADP- adenosine triphosphate a molecule that transfers energy for metabolic activities in cells
Note
0.0(0)
AP Biology Study Guides
AP Biology Ultimate Guide
Unit 1: Chemistry of Life
Unit 2: Cell Structure and Function
Unit 3: Cellular Energetics
Unit 4: Cell Communication and Cell Cycle
Unit 5: Heredity
Unit 6: Gene Expression and Regulation
Unit 7: Natural Selection
Unit 8: Ecology
Studying for another AP Exam?
Check out our other AP study guides