Anatomy Ch 2
Matter, Atoms, Elements, and the Periodic Table
Matter has mass and occupies space
3 forms of matter:
Solid (ex, bones)
Liquid (ex, blood)
Gas (ex, oxygen)
Atom - The smaller particle displays chemical properties of an element.
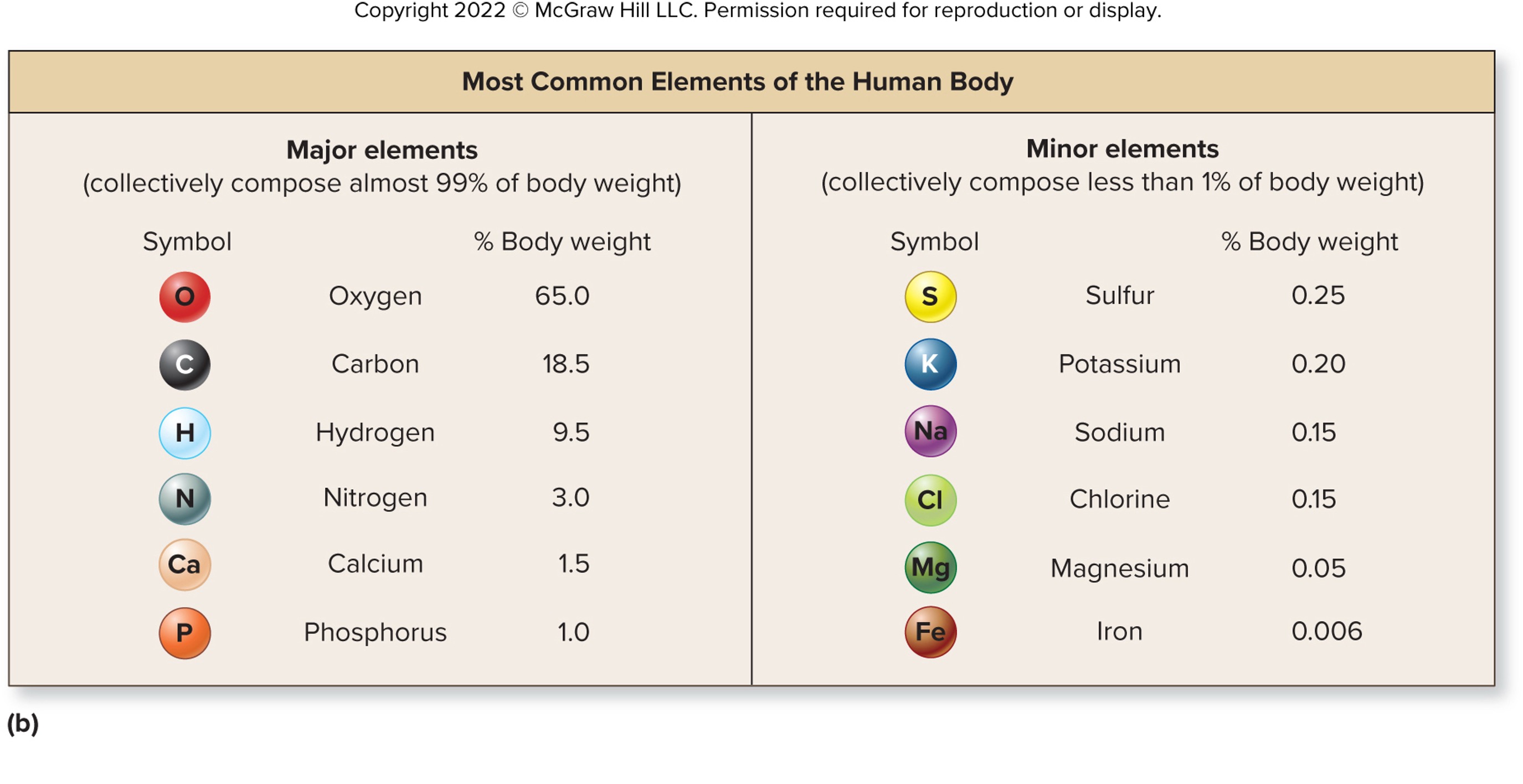
92 Naturally occurring elements make up matter
organized in the periodic table of elements
Chemical Formulas: Molecular and Structural
Molecular Formula
Indicates the number of types of atoms
ex: Carbonic Acid (H2CO3)
Structural Formula
shows the number and kind of atoms
shows the arrangement of atoms within the molecule
ex: O=C=O (carbon dioxide)
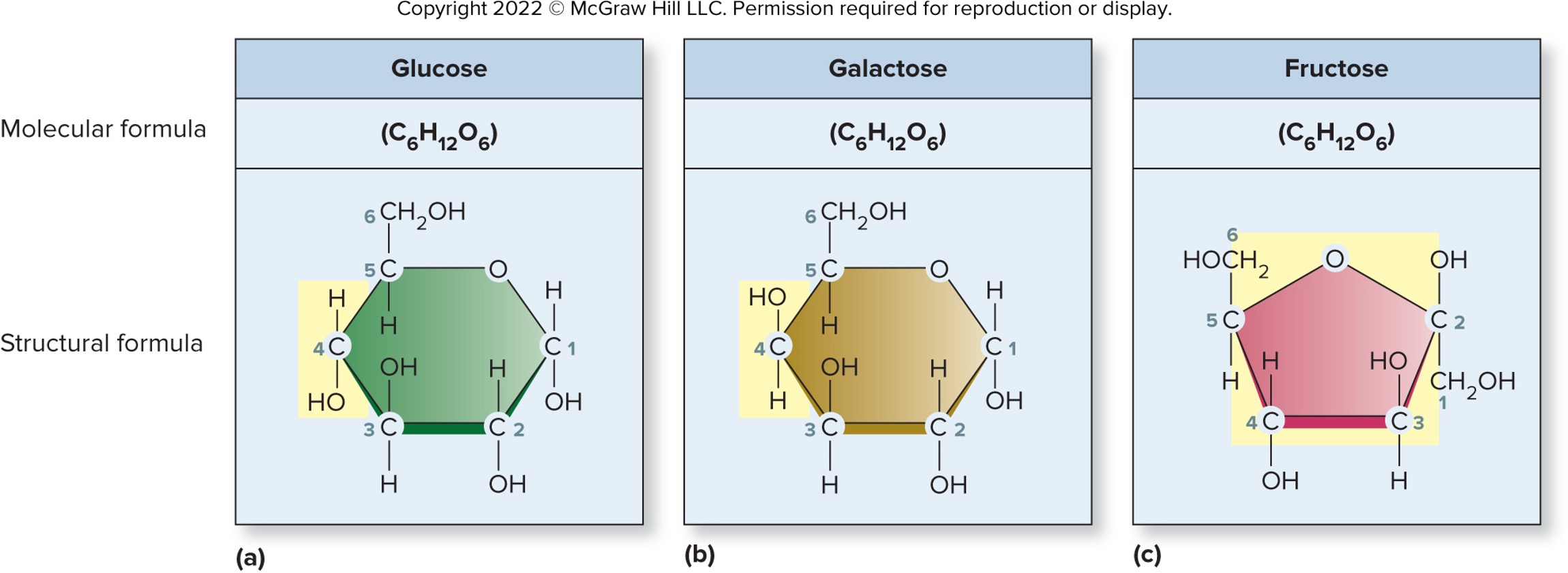
Allows differentiation of isomers
Same number and type of elements, but arranged differently in spaces
Glucose vs Galactose vs Fructose
Samww molecular formula
6 carbon, 12 hydrogen, 6 oxygen
Atroms arranged differently
Isomers may have different chemical properties
Covalent Bonds
Atoms share electrons
Occurs when both atoms require electrons
Formed commonly in the human body using
Hydrogen (H)
Oxygen (O)
Nitrogen (N)
Carbon (C)
Number of covalent bonds an atom can form:
The simplest occurs between 2 hydrogen atoms
each sharing a single electron
Oxygen needs two electrons to complete its outer shell
forms two covalent bonds
Nitrogen forms three bonds
Carbon forms four bonds
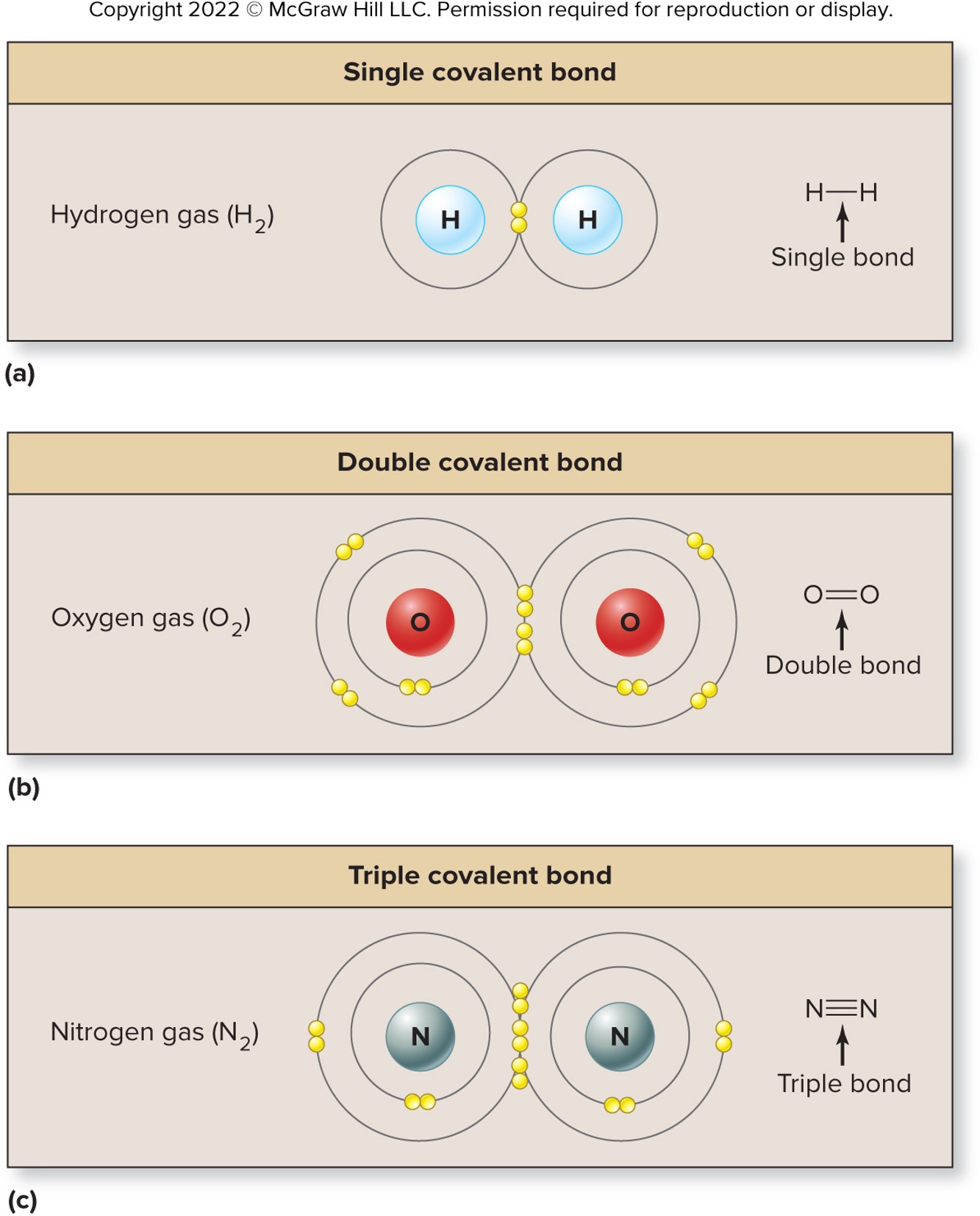
Single Covalent Bond
One pair of electrons is shared
ex: between two oxygen atoms
Double Covalent Bond
Two pairs of electrons are shared
ex: between two oxygen atoms
Triple Covalent Bond
Three pairs of electrons are shared
ex: between two nitrogen atoms
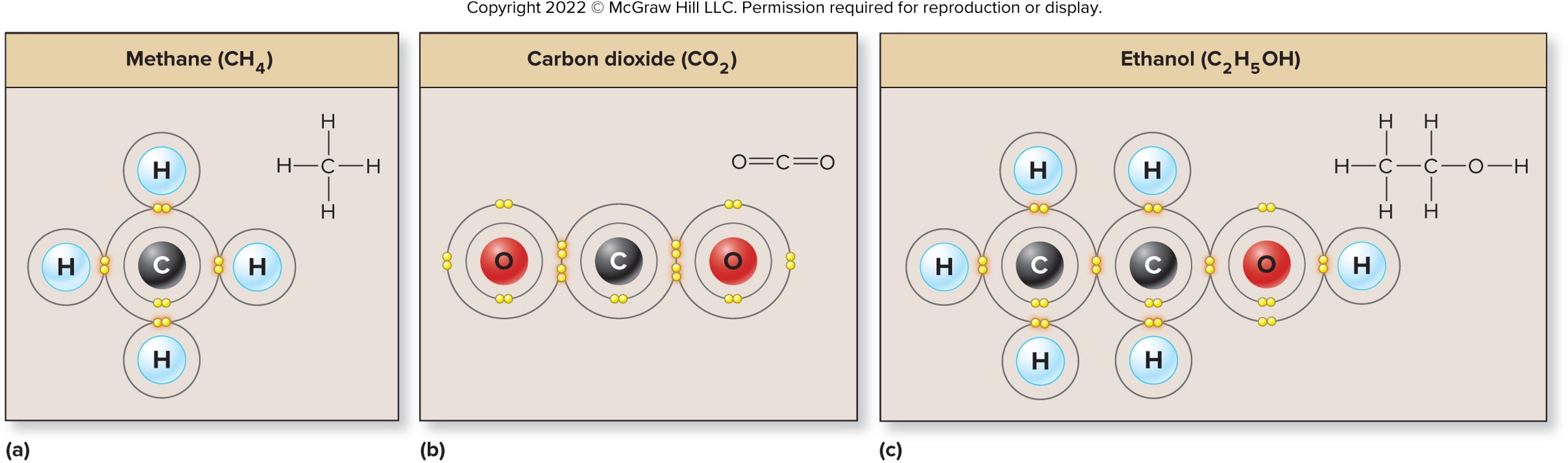
Carbon needs four electrons to satisfy the octet rule
can be obtained in several ways
Carbon Skeleton Formation;
Carbon
Bonds in straight chains, branched chains, or rings
called the carbon skeleton
Carbon is present where lines meet at an angle
Additional atoms are hydrogen
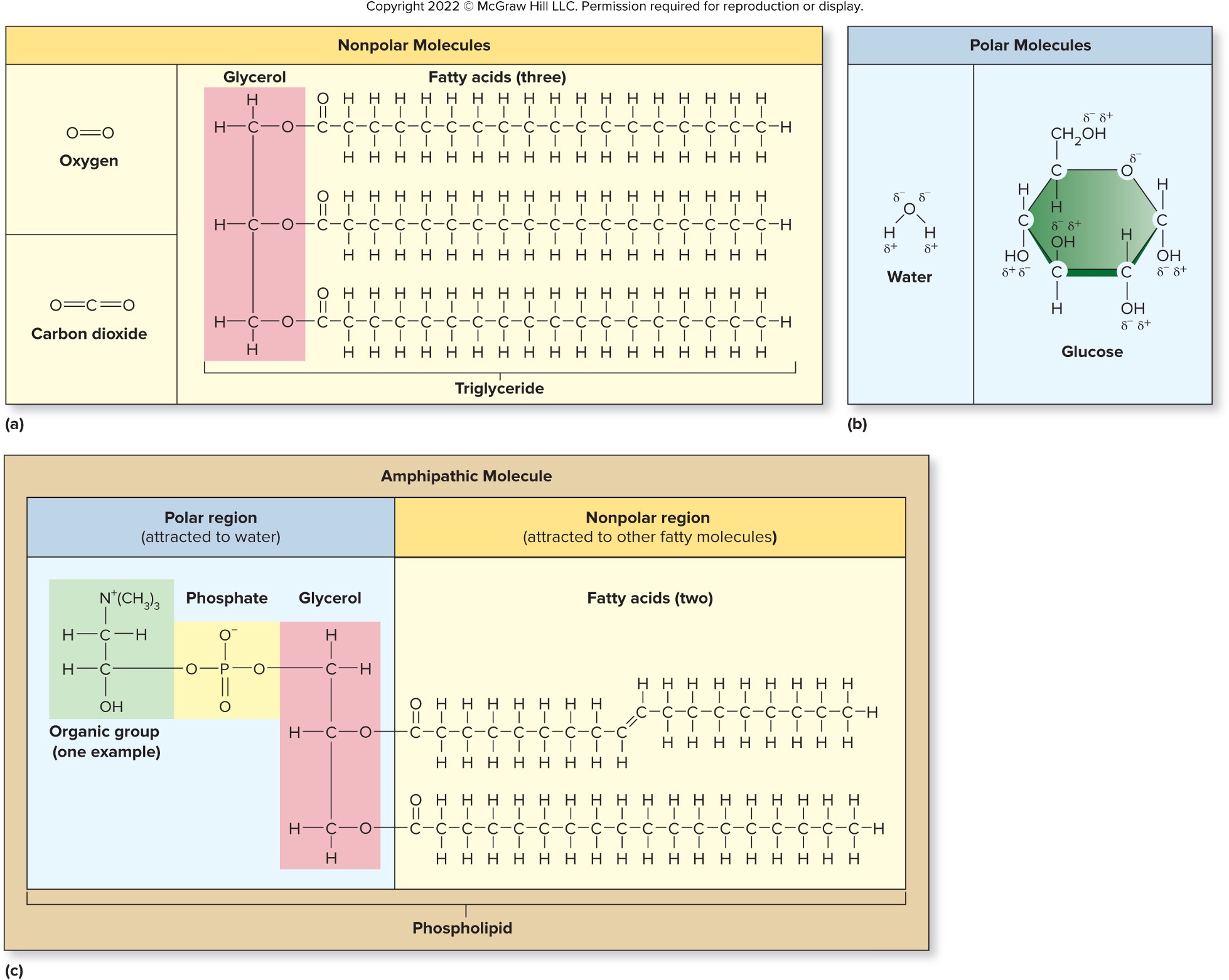
Nonpolar, Polar, and Amphipathic Molecules
Amphipathic molecules
larger molecules with both polar and nonpolar regions
ex: phospholipids
Molecular Structure and Properties of Water
Molecules are classified as:
Organic Molecules - contain carbon and are/were part of a living organism.
Inorganic Molecules - include all other molecules
ex: water, salts, acids, and bases
Water
Composes 2/3 of human body weight
Polar molecule
1 oxygen atom bonded to two hydrogen atoms
oxygen atom has two partial negative charges
hydrogens have a single partial positive charge
Can form 4 hydrogen bonds with adjacent molecules
central to water’s properties
Properties of Water
Phases of water depending on temperature:
Gas (water vapor)
Substances with low molecular mass
Liquid (water)
Almost all water in the body
liquid at room temp due to hydrogen bonding
Solid (ice)
Functions of Liquid Water
Treansports
Substances dissolved in water move easily through the body
Lubricates
Decreases friction between body structures
Cushions
absorbs the sudden force of body movement
Excretes Wastes
Unwanted substances that dissolve in water are easily eliminated.
Water as the Universal Solvent
Water is the solvent of the body
Solutes are the substances that dissolve in water
Water is called the universal solvent because most substances dissolve in it
chemical properties of a substance determine whether it will or won’t dissolve.
Substances that dissolve in water - polar molecules and ions
Hydrophilic means “water-loving” on
Water surrounds substances, forming a hydration shell
Some substances dissolve but remain intact
ex: glucose and alcohol
Nonelectrolytes remain intact but don’t conduct current
Some Substances dissolve and dissociate (separate)
NaCl dissociates into NA+ and Cl- ions
Acids and bases, like HCl
Electrolytes can conduct current
Substances that DON’T dissolve in water - Nonpolar molecules
Hydrophobic means “water fearing”
Hydrophobic exclusion - cohesive water molecules “force out” nonpolar molecules.
Excluded molecules interact via hydrophobic interactions
Hydrophobic substances require carrier proteins to be transported within the blood
Substances that partially dissolve in water
Amphipathic molecules have polar and nonpolar regions
The polar portion of the molecule dissolves in water
nonpolar portion repelled by water
Phospholipid molecules are amphipathic
Polar heads have contact with water
nonpolar tails group together
Results in bilayers of phospholipid molecules
ex: membranes of a cell.
Other amphipathic molecules form a Micelle.
Watee: A neutral Solvent
Water spontaneously dissociates to form ions
The bond between oxygen and hydrogen breaks apart spontaneously
1/10,000,000 ions per liter
OH group hydroxide ion (OH-)
A hydrogen ion is transferred to a second water molecule
Hydronium ion (H3O+)
An equal number of positive hydrogen ions and negative hydroxyl ions are produced
water remains neutral - H2O + H2O → H3O+ + OH−
simplifies to: H2O → H+ + OH–
Acids and Bases
Base accepts H+ when added to a solution
proton acceptor
deceased concentration of free H+
more absorption of H+ with stronger bases
ex: ammonia and bleach
Less absorption of H+ with weaker bases
ex: bicarbonate in blood and secretions released into the small intestine
Substance B ( a base in water) + H+ —→B—- H
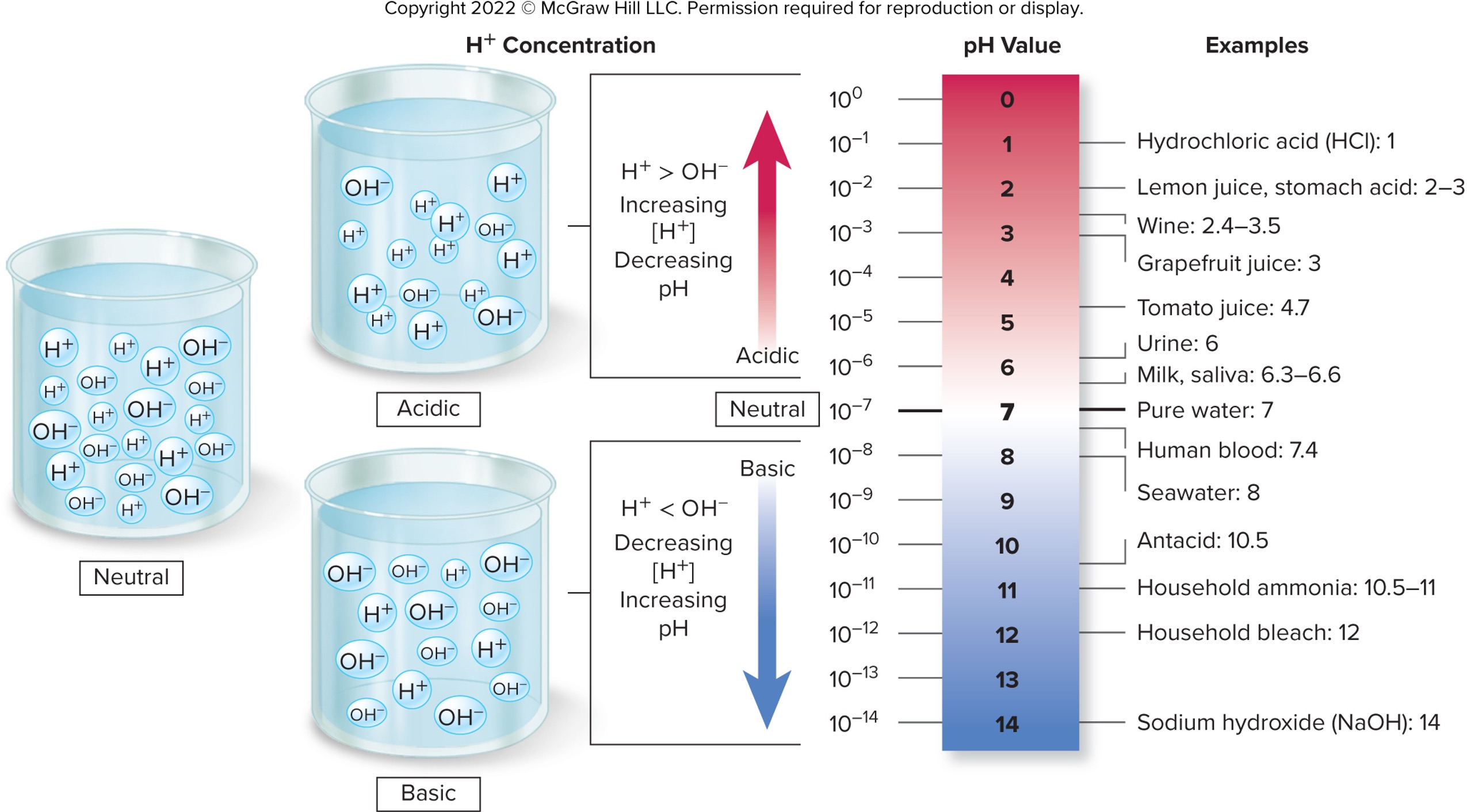
pH, Neutralization, and the Action of Buffers
pH is a measure of H+
relative amount of H+
Range between 0 and 14
The pH of plain water is 7
water dissociated to produce 1/10,000,000 of H+ and OH- ions per liter
equal to 1 times 10-7 or to 0.0000001
pH and H+ concentration are inversely related
Inverse of the log for a given H+ concentration
As H+ concentration increases, pH decreases
As H+ concentration decreases, pH increases
Interpreting the pH scale
Solutions with equal concentrations of H+ and OH−
Are neutral
Has a pH of 7
Solutions with greater H+ than OH−
Are acidic
Have a pH < 7
Solutions with greater OH− than H+
They are basic (alkaline)
Have a pH > 7
Moving from one increment to the next is a 10-fold change
ex: a pH of 6 has 10 times greater concentration of H+ than pure water
Neutralization
When an acidic or basic solution returns to neutral (pH 7)
Acids are neutralized by adding a base
ex: medications to neutralize stomach acid must contain a base
Bases neutralized by adding acid
Buffers
Help prevent pH changes if excess acid or base is added
Act to accept H+ from excess acid or donate H+ to neutralize base
carbonic acid (weak acid) and bicarbonate (weak base) buffer blood pH
Both help maintain blood pH in a critical range (7.35 to 7.45)
Lipids
diverse group of fatty, water-insoluble molecules
function as stored energy, cellular membrane components, and hormones.
Four primary classes:
Triglycerides
phospholipids
eicosaniods
Triglycerides are used for long-term energy storage
Formed from glycerol and 3 fatty acids
Fatty acids vary in length and number of double bonds
Saturated - lack double bonds
Unsaturated - one double bond
Polyunsaturated - two or more double bonds
Adipose tissue stores triglycerides
Lipogenesis - formation of triglycerides when there’s excess nutrients
Lipolysis - breakdown of triglycerides when nutrients are needed
Fatty Acids: Saturated, unsaturated, and trans fats
Most animal fats are Saturated
Most are solid at room temperature
Most vegetable fats are unsaturated
Most are liquid at room temperature
generally healthier
can be converted to saturated fats through hydrogenation
Partial hydrogenation may lead to Trans Fats
increase the risk of a heart attack and stroke
Carbs
Carbohydrates
An- H and an -OH are usually attached to every carbon
The general chemical formula is (CH2O)n
n = number of carbon atoms
Monosaccharides
Simple sugar monomers
Disaccharides
formed from two monosaccharides
Polysaccharides
formed from many monosaccharides
Glucose
6 carbon carb
Most common monosaccharides
primary source of energy to cells
Concentration must be carefully maintained
Glycogen
Liver and skeletal muscle store excess glucose and then bind glucose monomers together (glycogenesis)The liverr hydrolyzes glycogen into glucose as needed (glycogenolysis)
The liver can also form glucose from non-carbohydrate sources (gluconeogenesis)
Nucleic Acids
Store and transfer genetic information
two classes of nucleic acid
Deoxyribonucelic acid (DNA)
Ribonuecleic Acid (RNA)
both are polymers composed of nucleotide monomers
Monomers are linked covalently through phosphodiester bonds.
Deoxyribonucleic acid (DNA)
Double-stranded nucleic acid
Located in chromosomes in nucleus and in mitochondria
Deoxyribose sugar, phosphate, and one of four nitrogenous bases
Adenine, guanine, cystosine, thymine
No uracil
Double strands held together by hydrogen bonds
Form between complementary bases
Thymine paired with adenine
Guanine paired with cytosine
Ribonucleic acid (RNA)
Single-stranded nucleic acid
Located in nucleus and in cytoplasm of cell
Ribose sugar, phosphate, and one of four nitrogenous bases
Adenine
Guanine
Cystosine
Uracil
No thymine
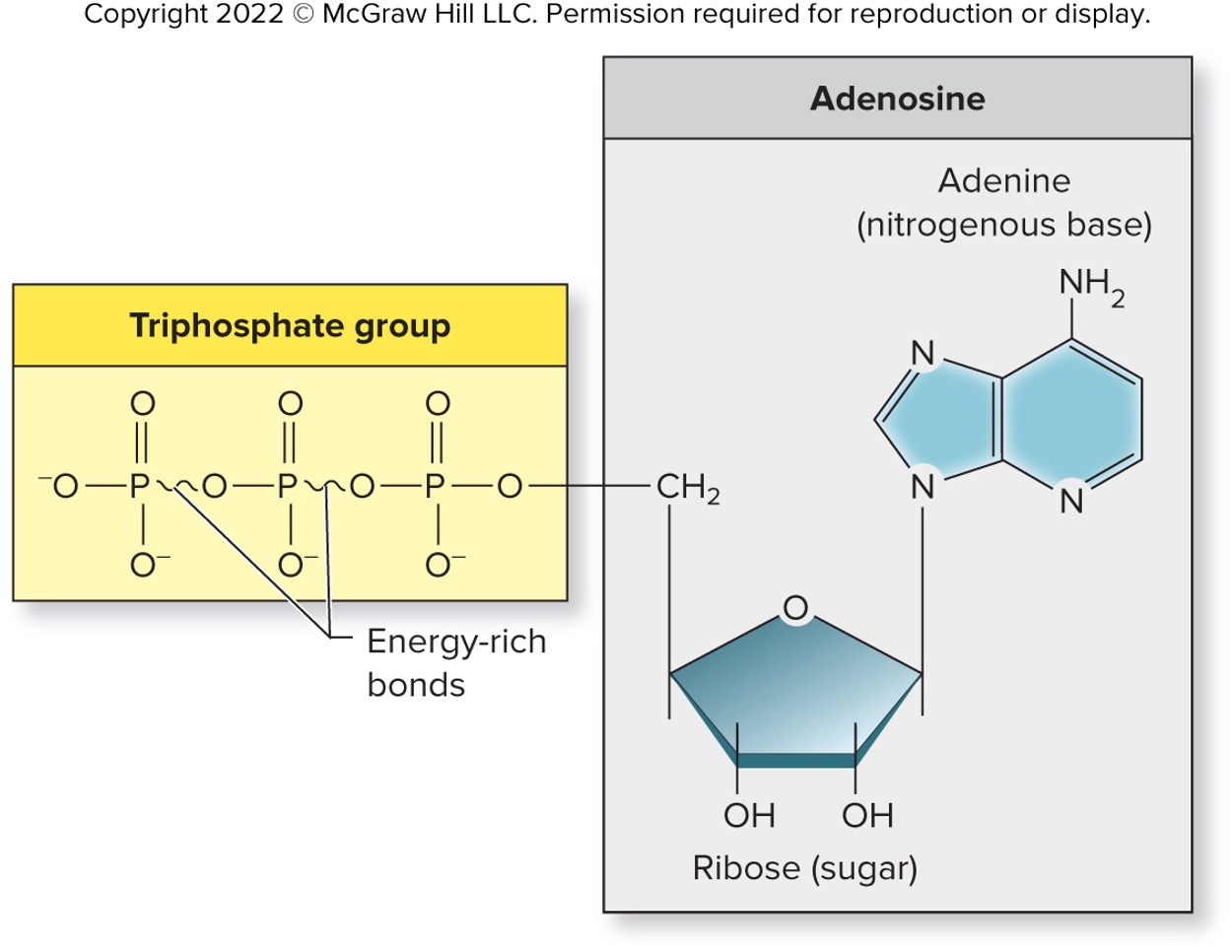
Adenosine triphosphate (ATP)
A nucleotide is composed of a nitrogenous base adenine, a ribose sugar, and three phosphate groups
Central molecule in the transfer of chemical energy within cell
Covalent bonds between the last two phosphate groups are unique,
energy richRelease energy when broken, Important nucleotide-containing molecules
Nicotinamide adenine dinucleotide
Flavin adenine dinucleotide
Both participate in production of ATP
Proteins
Protein functions include
Synthesis and digestion (actions of enzymes)
Structural support. ex: cytoskeleton proteins
Body movement. ex: actin and myosin of muscle
Transport in blood; ex: hemoglobin carries O2
Membrane transport via carrier proteins
Protection, ex: antibodies