AP Biology Notes/Review <3 (ALL 8 UNITS)
Unit 1 - Water and Life ✓
https://docs.google.com/presentation/d/11PdEfOsShrBiYMjP0EEweFGjjrDLUU9pTHIeY_lkMmo/edit#slide=id.p2
Water
Makes life possible on Earth
Organisms are mostly made of water
Liquid, solid, or gas
Hydrogen Bonding
Because oxygen is more electronegative, it hogs electrons, making H2O a polar covalent bond
Oxygen has a partial negative charge (𝛿-) and hydrogen has a partial positive charge (𝛿+)
Water molecules will form hydrogen bonds with one another
Oxygen attracted to the Hydrogen (diff water molecules)
Hydrogen bonds between water molecules are constantly breaking and reforming
Hydrogen bonds are ~ 1/20 the strength of covalent bonds
Properties of Water
4 important properties of water that make it so good for supporting life
Cohesion
Temperature moderation
Floating of ice on liquid water
Use as a solvent
Cohesion hydrogen bonds hold water molecules together
Helps plants to transport water up through their roots against gravity
Water evaporates from leaves → more water moves up the vessels in the plant
Causes water molecules to pull other water molecules up the plant
Adhesion, the phenomenon of one substance sticking to another, also helps the water cling to the sides of the vessels
Surface Tension is the measure of the force necessary to stretch or break the surface of a liquid
Water has a large surface tension because its hydrogen bonds resist stretching and breaking
How mosquitoes and other animals are able to stand on water
Water’s High Specific Heat is the measure of how much heat it takes to cause a substance to change its temperature
Due to hydrogen bonds
A large body of water can absorb a large amount of heat from the sun and only get warm a couple of degrees
Coastal area = stable temperature
Because living things are composed largely of water, they resist temperature change well
Heat of Vaporization the quantity of heat a liquid must absorb for one gram of it to be converted from liquid to gas
Evaporation = liquid is transformed into a gas
Water = high heat of vaporization
This is due to the need to break hydrogen bonds
between water molecules to convert the water
to a gasHelps moderate the climate
Lots of the sun’s heat gets absorbed by water, moderating the temperature
Evaporative Cooling as liquid evaporates, the surface of the liquid left behind is cooled
The most energetic molecules, therefore the ones with the most heat, are the most likely to evaporate
Mammals utilize evaporative cooling in the form of sweating
Plants utilize evaporative cooling as water evaporates from their leaves
Floating Ice
Oceans and lakes don’t freeze solid because ice freezes on liquid water
Ice is less dense than water, due to the fact that the water molecules are locked into a crystal lattice when frozen
The frozen ice on top of the cold liquid water insulates the water below, allowing life to flourish below
Water as a solvent
Solution a liquid that is a completely homogenous mixture of two or more substances
Solvent what the dissolving
Solute is a substance that is dissolved
Aqueous solution in which water is the solvent
Water is a very versatile solvent due to the fact that water molecules are polar
Each dissolved ion is surrounded by water molecules, forming a hydration shell
Hydrophilic water loving
Substances have lots of ionic and polar bonds, making them able to interact well with water
Hydrophobic water-fearing/hating
Substances that are nonionic and have nonpolar bonds
Water molecules can’t form hydrogen bonds with hydrophobic substances
Oils are hydrophobic because it’s nonpolar
Wet Chemistry
Called this due to reactions involving solutes dissolving in water
Chemical reactions depend on the concentration of different solutes in water
Molarity the concentration of a material in a substance (M)
A solution with a molarity of 1 has one mole of substance dissolved in 1 liter of water
Hydrogen Ions
H2O ←→ H- + OH-
This dissociation is very rare, but very important in the chemistry of life
H+ and OH- are very reactive and can drastically affect the chemistry of the cell
The concentration of H+ and OH- affects the pH of a solution
Acids and Bases
The pH scale measures how acidic or basic a solution is
Acids are numbers below 7 on the pH scale
Bases are numbers above 7 on the pH scale
Acids are substance that increases the hydrogen ion concentration of a solution
The more H+ in a solution, the more acidic it is
When HCl (hydrochloric acid) is added to a solution, hydrogen ions dissociate from chlorine ions, adding H+ to the solution
HCl + H+ → Cl-
Bases substance that reduces the hydrogen ion concentration of a solution is a base
The less H+ in a solution, the more basic it is
Some bases directly reduce the H+ concentration by accepting hydrogen ions
NH3 + H+ ←→ NH4+
Organisms and pH
Human blood is pH 7.4, if it changes the person will die
Cells use buffers to keep their pH levels within a comfortable range
Temperature Moderation
Atoms and molecules have kinetic energy due to the fact that they’re always moving
Heat the measure of the total kinetic energy due to molecular motion in a body of matter
Temperature the intensity of heat in a body of matter due to the average kinetic energy of molecules
When two objects of different temperatures come together, heat passes from the warmer object to the cooler object until they’re the same temperature
Unit 1 - Organic Molecules ✓
https://docs.google.com/presentation/d/13LxD5zBm_mS5Qh6AlSzOYfsVi3uBHVcZ7S8M0KPSaI8/edit#slide=id.p1
Macromolecules are composed of small organic molecules
Make up all living things
4 main classes
Carbohydrates
Lipids
Proteins
Nucleic acids
Polymers and Monomers
Polymer is a long molecule composed of many similar building blocks linked by covalent bonds
Poly = many
Monomers are the small building blocks that compose polymers
Mono = one
Making and Breaking Polymers
All cells use similar processes to assemble and break apart macromolecules
These processes are facilitated by enzyme
Dehydration reactions build polymers
Hydrolysis breaks polymers
Dehydration Reaction links monomers together to form polymers
Both monomers contribute part of a water molecule
One monomer contributes an OH group
The other contributes an OH
Product = water
Hydrolysis breaks apart polymers
Bonds are broken apart by the addition of water molecules
A hydrogen attaches one monomer and an OH attaches to the other monomer
Utilized by the body during digestion
Carbohydrates
Include sugars and their polymers
Monosaccharides are the simplest carbohydrates
Disaccharides are composed of two monosaccharides
Polysaccharides are polymers of many monosaccharides
Monosaccharides are the monomers for carbohydrates
Their molecular formulas are typically CnH2nOn
Glucose is C6H12O6
Most sugar names end in –ose
Monosaccharides are classified by the
number of carbons in their carbon skeletonCarbon skeletons range from 3-7 carbons long
6-carbon sugars like glucose are hexoses
Most sugars form rings when in an aqueous solution
Monosaccharides are used as a major source of nutrients for cellular work
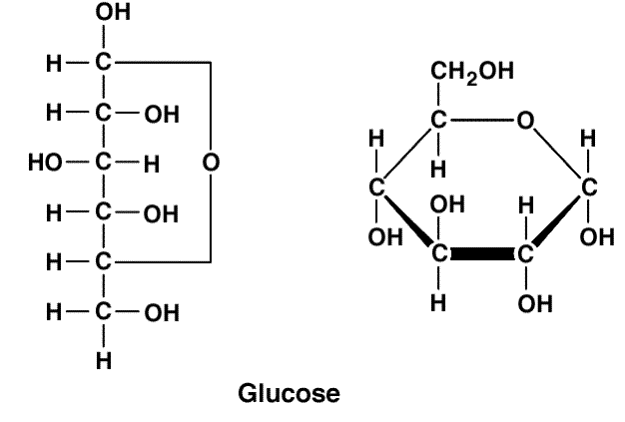
Glycosidic Linkage
Two monomers can join together with a glycosidic linkage to form a disaccharide
A disaccharide is two monomers linked together
Maltose is formed by joining two glucose molecules
Sucrose is formed by joining fructose and glucose
Lactose is formed by joining glucose and galactose
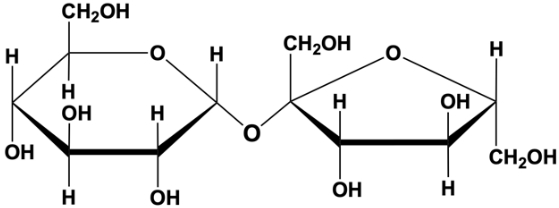
Polysaccharides are composed of hundreds of monosaccharides
Some are used as energy reserve
Others are used as building materials
Starch is a storage polysaccharide used by plants
Composed entirely of glucose monomers
Plants store glucose as starch and draw upon it as needed for energy
Glycogen animals store glucose in a polysaccharide
Glycogen is a highly branched molecule
Vertebrates store glycogen in the liver and muscles and break it down to release glucose
Cellulose is a polysaccharides that helps to make up cell walls in plants
Humans can’t digest
Many eukaryotic herbivores have a symbiotic relationship with cellulose digesting microbes
Polysaccharides Shapes
Differences in glycosidic linkages contribute to different shapes in polysaccharides
A β glucose is one that is above the plane of the ring
An α glucose is one that below the plane of the ring
Differences in the positioning of glucoses in polysaccharides affects the shape of the polysaccharide
Alpha and Beta Linkages
Starch is composed mainly of alpha linkages
This gives starch a more helical shape and allows it to be branched
Ex) Glycogen
Cellulose is made up of beta linkages
This gives cellulose a more linear shape
Because of this, cellulose strands are able to bond with adjacent strands, strengthening the fibers
Humans can’t digest these beta linkages
Chitin is a structural polysaccharide
Found in exoskeletons of arthropods and in the cell walls of fungi
Similar to cellulose
Chitin has β linkages
Lipids are often referred to as fats or waxes
Have little or no affinity for water because they are nonpolar
Useful both as a long-term energy source and structurally
Fats store large amounts of energy
Composed of glycerol and fatty acids
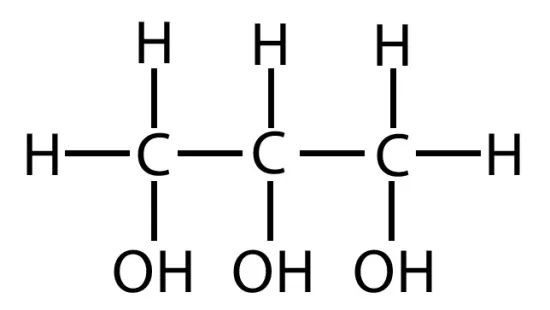
Glycerol is a 3 carbon alcohol
A fatty acid consists of a carboxyl group attached to a long carbon skeleton
Triglycerides (triglycerol) is composed of a glycerol bound to 3 fatty acids
Fatty acids can be the same or different
Fatty acids are joined to the glycerol by dehydration synthesis
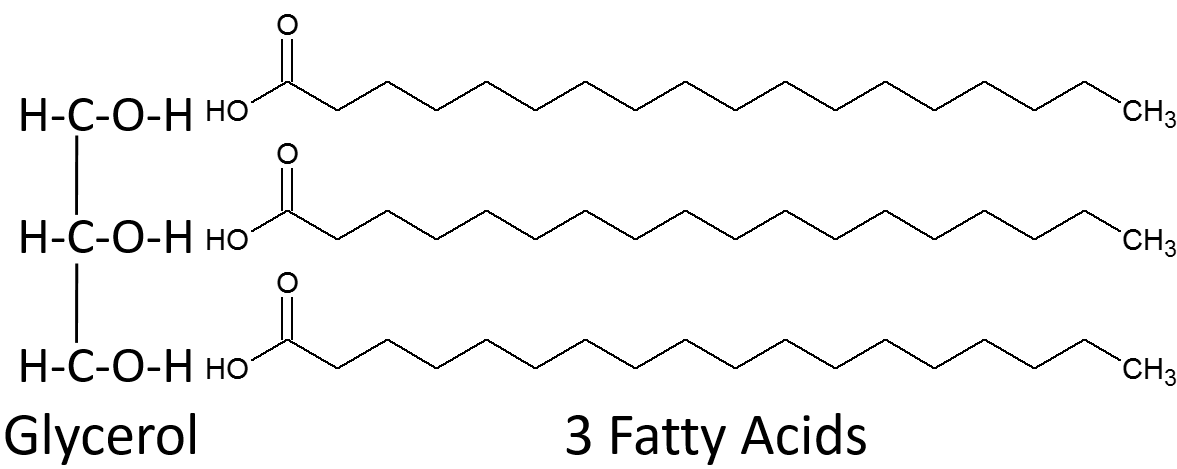
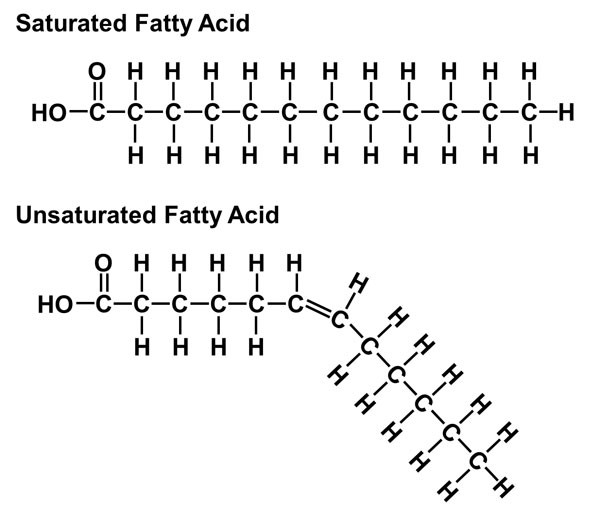
Saturated Fatty Acids if there are no carbon-carbon double bonds in the fatty acid
It’s saturated with hydrogens in every possible position
Solid at room temperature and can lead to cardiovascular diseases
Unsaturated Fatty Acids have one or more double bonds in the carbon skeleton
Have a kink in the carbon skeleton
These fats are liquid at room temperature and aren’t as unhealthy
Fat Energy Storage to store energy
A gram of fat stores more than twice the amount of energy as a gram of carbohydrate
Plants use fat storage in the form of oils in seeds
Humans and other mammals store fats as long term energy reserves in adipose cells
Phospholipids is composed of a glycerol attached to a phosphate and two fatty acids
Cell membranes are made largely of phospholipids
The phosphate head of the phospholipid is hydrophilic
The fatty acid tails of the phospholipid are hydrophobic
Phospholipids arrange themselves as a bilayer when exposed to water
The tails of one each side of the layer point in to avoid the water
The heads of the phospholipids point out towards the water
This forms the main component of cell membranes
Steroids are lipids with a carbon skeleton composed of four fused rings
Cholesterol is a steroid found in animal cell membranes
High levels of cholesterol can contribute to cardiovascular disease
Testosterone and estrogen hormones are steroids
Proteins
Functions including:
Structural support
Storage
Transport
Defense
Movement
Protein enzymes function as catalysts in cells
Polypeptides make up proteins
Amino acids make up polypeptides
Amino Acids are the monomers of proteins (they contain both carboxyl and amino groups)
The amino acid is composed of 4 things attached to a central carbon atom:
A hydrogen atom
A carboxyl group
An amino group
A variable R group
R Group determines the characteristic of the amino acid
Each 20 amino acid has its own R group
R groups can be:
Polar
Nonpolar
Negatively Charged
Positively Charged

Joining Amino Acids together by dehydration synthesis
The covalent bond that links amino acids together is called a peptide bond
When many amino acids are linked together, they form a polypeptide chain
The N-terminus of the chain has a free amino group
The C-terminus of the chain has a free carboxyl group
Protein Formation
A protein consists of one or more polypeptides arranged into a unique shape
The order of amino acids determines the shape of the protein
The shape of the protein spontaneously forms due to the interactions of the polypeptide chain with itself
Different amino acids on the chain bind to one another, reinforcing the shape of the chain
The shape of the protein determines its function
Primary Structure protein is its unique sequence of amino acids
This is just the amino acids that make up the polypeptide chain and the order in which they’re found
A slight change in primary structure can have large repercussions
Secondary Structure the coils and folds in the polypeptide chain that give the chain shape
Weak hydrogen bonds between many amino acids in the chain hold the secondary structure together
Each hydrogen bond is weak individually, but together, they’re strong
α helix is a secondary structure in which the polypeptide chain coils
Hydrogen bonds between every 4th amino acid hold the structure together
β pleated sheet is a secondary structure
In a pleated sheet, multiple regions of the chain lying side by side are connected by hydrogen bonds between parts of the two parallel polypeptide backbones
Tertiary Structure is the 3D shape of the entire protein
Determined by interactions among various R groups including:
Hydrophobic interactions
van der Waals interactions
Ionic bonds
Hydrogen bonds
Disulfide Bridges
Strong covalent bonds between sulfhydryl groups of cysteine amino acids help to hold the 3D shape of the protein
These bonds are called disulfide bridges because two sulfides connect to one another
Quaternary Structure results from the aggregation of two or more polypeptides
This is when a protein is made of multiple polypeptides
Hemoglobin is a protein with a quaternary structure
It’s composed of 4 different
polypeptides
Denaturation
Protein structure depends on the physical and chemical conditions of the protein’s environment
Alterations in pH, salt concentration, or other factors can denature a protein by disrupting bonds that hold it together
When a protein is denatured , its shape is changed and it can’t function as normal
Denatured proteins are biologically inactive
Chaperonins help unfold proteins
Protect the polypeptide while it’s folding
Misfolded proteins can be fixed by the chaperonins
Misfolded proteins can lead to diseases such as Alzheimer's, Parkinson’s, and mad cow disease
Nucleic Acids
Genes
The amino acid sequence of a polypeptide is decoded by a gene
A gene is composed of DNA
Nucleic acids include DNA and RNA
Nucleic acids are made up of monomers called nucleotides
RNA and DNA
DNA encodes for proteins that make up the body
Found in the nucleus
RNA takes the information from DNA and uses it to make proteins
Messenger RNA is made by reading DNA and encodes for proteins
Protein synthesis occurs on ribosomes
Nucleotides
Nucleic acids are polymers that are made up of nucleotides
Each nucleotide is composed of 3 parts:
A nitrogenous base
A, T, C, G, or U
A pentose sugar
A phosphate group
Pyrimindes and Purines
Nitrogenous bases can be classified as purines or pyrimidines
Pyrimidines have single six-membered rings composed of carbon and nitrogen
Cytosine (C), Thymine (T), and Uracil (U)
Purines have a six-membered ring
joined to a five-membered ringAdenine (A) and Guanine (G)
Nucleotide Pentose
The pentose in RNA is ribose
The pentose in DNA is deoxyribose
The only difference between the two is that deoxyribose lacks an oxygen atom on carbon 2
Polynucleotides are created when nucleotides are joined by covalent bonds
The bonds that join nucleotides are called phosphodiester bonds
The OH group on the 3’ end of one nucleotide links to the phosphate on the 5’ end of the other
DNA Double Helix (shape of DNA)
The sugar-phosphate backbones of the two polynucleotides are on the outside of the helix
The backbones run in opposite 5’ —> 3’ directions
Their arrangement is said to be antiparallel
The strands are held together by hydrogen bonds between the paired bases
Adenine pairs with thymine
2 hydrogen bonds between the two
Guanine pairs with cytosine
3 hydrogen bonds between the two
The two strands are complementary
to one another
DNA Replication each strand acts as a template to order nucleotides in a new complementary strand
This ensures that the correct set of genetic information is transmitted when a cell reproduces
RNA
Complementary pairing is also important in RNA
Transfer RNA delivers its amino acids based on base pairing
In RNA, there is uracil instead of thymine
It pairs with adenine
RNA is typically single-stranded
Unit 2 - Cells
https://docs.google.com/presentation/d/1zkrfYmZHKU_6nKkKX7z3zw5-TKmEu7AzexH9UCnO2GE/edit#slide=id.p1
Cells
All organisms are made of cells
The cell is the simplest collection of matter that can be alive
Cells are the basic building block of life
Microscopy
The development of microscopes allowed for the discovery of cells
In light microscopes, visible light passes through the specimen and through the glass lenses
These can distinguish between individual cells, but can’t see the organelles
In electron microscopes, a beam of electrons passes through the specimen
These can see smaller things such as organelles
Electron Microscopy
Scanning electron microscopes (SEMs) are used for studying the surface structure of a specimen
The image seems three-dimensional
Transmission electron microscopes (TEMs) are used to study the internal structure of cells
Magnification – the ratio of an object’s image to real size
A light microscope can magnify to about 1000x
Resolution – a measure of image clarity
Contrast – accentuates differences in parts of the sample
The higher the contrast, the easier it is to tell the two things apart
Cell fractionation is a technique that takes cells apart and separates major organelles and other subcellular structures
A centrifuge spins test tubes with mixtures of disrupted cells
Different organelles settle into pellets at the bottom of the test tube at different speeds
PROKARYOTIC VS. EUKARYOTIC CELLS
Bacteria and Archaea have prokaryotic cells
Prokaryotic cells are between .1µm and 5 µm
Protists, fungi, animals, and plants have eukaryotic cells
Eukaryotic cells are between 10 and 100 µm
Eukaryotes house their DNA in a membrane-bound nucleus
Prokaryotes don’t have a
nucleus, they have a
nucleoid region
Eukaryotic cells have membrane-bound organelles, while prokaryotes
don’t
Unit 2 - Cells Transport
https://docs.google.com/presentation/d/1fFcBwFSwcY4VL2suQwtU6JtpkgKfr7a-q5lWrWVjWww/edit#slide=id.p1
Unit 3 - Enzymes
https://docs.google.com/presentation/d/1GpAmcUCyzF8BzLc1DAr2Qw7q9KbnuBiNgO7r3MRmg7k/edit#slide=id.p1
Unit 3 - Photosynthesis
https://docs.google.com/presentation/d/1BIO8OlZm525FPklZ0-gGzn2s12N7hV0NYYrnU2_hHvU/edit
Unit 3 - Cellular Respiration
https://docs.google.com/presentation/d/1SncHcomeGN95F8DZ3cFShIfnuHiEpkjE3bI98fMH48M/edit
Unit 4 - Cell Communication
Unit 4 - Cell Cycle
https://docs.google.com/presentation/d/1Yaox5vSTWaESgRChLJ8_obmjPq1Shmen00OldD1Fumo/edit#slide=id.p1
Unit 5 - Meiosis
https://docs.google.com/presentation/d/1pxKHZtOqSLNL_lcUg6fsHvaN7VGDq93g0E_IhK9waH0/edit#slide=id.p1
Unit 5 - Genetics
https://docs.google.com/presentation/d/1J2YSDfG8vxATLm31XWnyWWzOZp0nF12Q5lPUNwGaeJ0/edit
Unit 6 - DNA
https://docs.google.com/presentation/d/1CfKKUX2rLSbYSQ5TjF6Duf9xjnT6rai-gyjMYaDV7jI/edit#slide=id.p1
Unit 6 - Protein Synthesis
https://docs.google.com/presentation/d/1blA4Wq_4YsMshR7qqf8x7RpXOgl9-vxS9kroY-kCXvg/edit
Unit 7 - Natural Selection ✓
Hardy Weinberg
Hardy Weinberg Equilibrium describes a non-evolving population
It states that the frequencies of all alleles and genotypes in the population in the population
Equation
p + q = 1
p = frequency of the dominant allele
q = frequency of the recessive allele
p² + 2pq + q² = 1
p² = frequency of homozygous dominant genotype
2pq = frequency of heterozygous genotype
q² = frequency of homozygous recessive genotype
Hardy-Weinberg has 5 assumptions
For a population to remain in Hardy-Weinberg equilibrium, the genes of the population must be unchanging
Very large population size
In small populations, chance fluctuations in the gene pool will cause allele frequencies to change over time
No migration
For the frequency of genes to remain the same, there must be no new genes entering the population via immigration and none leaving via emigration
No net mutations
The gene pool is modified if mutations occur
Random mating
If non-random mating occurs, some traits will be passed on with greater frequency than others
No natural selection
If natural selection occurs, favorable genes will be passed on with greater frequency
Assumptions of Hardy-Weinberg
Whenever a population is violating a tenant of Hardy-Weinberg, it’s evolving
Does not occur in nature
Speciation and Trees
https://docs.google.com/presentation/d/1CtRC3gzqZGYNaWf3mCEwexVQahBWXzTeY9LU1E2xWRo/edit#slide=id.p1
Speciation the process by which one species splits into two or more species
Bridges the gap between microevolution and macroevolution
Biological species concept defines a species as a group of populations that have the potential to interbreed in nature and produce viable, fertile offspring
Different species are separated by reproductive isolation
Reproductive isolation is the existence of biological barriers that prevent members of two species from producing viable, fertile offspring
Prezygotic and Postzygotic Barriers
Prezygotic barriers block fertilization between species
A zygote never forms
Either mating doesn’t occur, or something hinders fertilization
Postzygotic barriers prevent the hybrid zygote from developing into a viable, fertile adult
Prezygotic Examples
Habitat Isolation two organisms that use different habitats are unlikely to encounter each other to even attempt mating
Behavioral Isolation species use unique and elaborate courtship behaviors to attract mates
Temporal Isolation two species that breed during different times of day, different seasons, or different years cannot mix gametes
Ex: The western skunk mates in the summer and the eastern skunk mates in the winter
Mechanical Isolation closely related species may attempt to mate, but fail because they are anatomically incompatible and the transfer of sperm isn’t possible
Snails whole shells coil in opposite spirals and cannot mate with each other
Gametic Isolation some of the gametes of two species do not form a zygote because of incompatibilities preventing fertilization
The female reproductive tract may be incompatible, the sperm and egg may not recognize each other, etc.
Postzygotic Barriers
Reduced Hybrid Viability genetic incompatibility between the two species may abort the development of the hybrid at some embryonic stage or produce frail offspring
Occasional hybrids form by salamanders that belong to the same genus
Reduced Hybrid Feritlity even if the hydrib offspring are vigorous, they’re infertile
When a donkey and a horse mate, they produce a mule
Hybrid Breakdown first-generation hybrids are viable and fertile, but the next generation is feeble or sterile
Some strains of rice have viable hybrids, but the next generation of hybrids are small and sterile (speciation)
Reproductive Barriers keep different species separate from each other
If two populations can’t interbeed, they’ll start to diverge into different species
Alternative Species Concepts
Limitations of the Biological Species Concept
Can’t test for extinct organisms (cuz they r dead <3)
Can’t test for organisms that reproduce asexually
Ecological Species Concept defines a species in terms of its ecological niche
Cosiders the sum of how members of the species interact with the nonliving and living parts of their environment
Can accommodate asexual and sexual species
Phylogenetic Species Concept defines species based one a shared common ancestor (the stupid tree <3)
A single branch in the tree of life is a species
Morphology and molecular sequences of species are analyzed to determine the evolutionary history of a species
Modes of Speciation
Allopatric Speciation geographic separation of populations restrict gene flow
A population is fragmated by a geographical feature into two or more isolated populations
Because the two populations are reproductively isolated, they diverge evolutionarily
Sympatric Speciation speciation occurs in populations that live in the same geographic area
Occurs by:
Polyploidy
Natural Selection
Sexual Selection
Artificial Selection
Polyploidy
Sympatric speciation can result from accidents in cell division that result in extra sets of chromosomes
Most common in plants (bc it can self-fertilize, easier to create new species)
Natural Selection
Diversifying selection can lead to sympatric speciation
If both extremes are selected for in a population, it can cause it break into two new species
Sexual Selection mates are chosen based on physical appearance
Can also lead to sympatric speciation
Tempos of Speciation
Gradualism a species changes slowly over time
It’s difficult to determine when a new species arises, as the change is slow (takes many generations)
Punctuated Equilibrium there are long periods of stasis followed by periods of rapid change
Suppose that a species survived for 5 million years, but most of its morphological alterations occurred in the first 50,000 years of its existence—just 1% of its total lifetime (still takes a long time, but it’s quicker)
Patterns of Evolution
Divergent Evolution occurs when a population becomes isolated from the rest of the species, becomes exposed to new selective pressures, and evolves into a new species
How new species arise
A new species diverges from the original species
Convergent Evolution occurs when unrelated species occupy the same environment, are subjected to similar pressures, and evolve in similar ways
The two unrelated organisms start to resemble each other and show similar adaptations
Analogous Structures are structures that have similar functions, but different evolutionary histores (ex. wings in butterflies and birds)
Parallel Evolution two species make similar evolutionary adaptations after divergence from a common ancestry
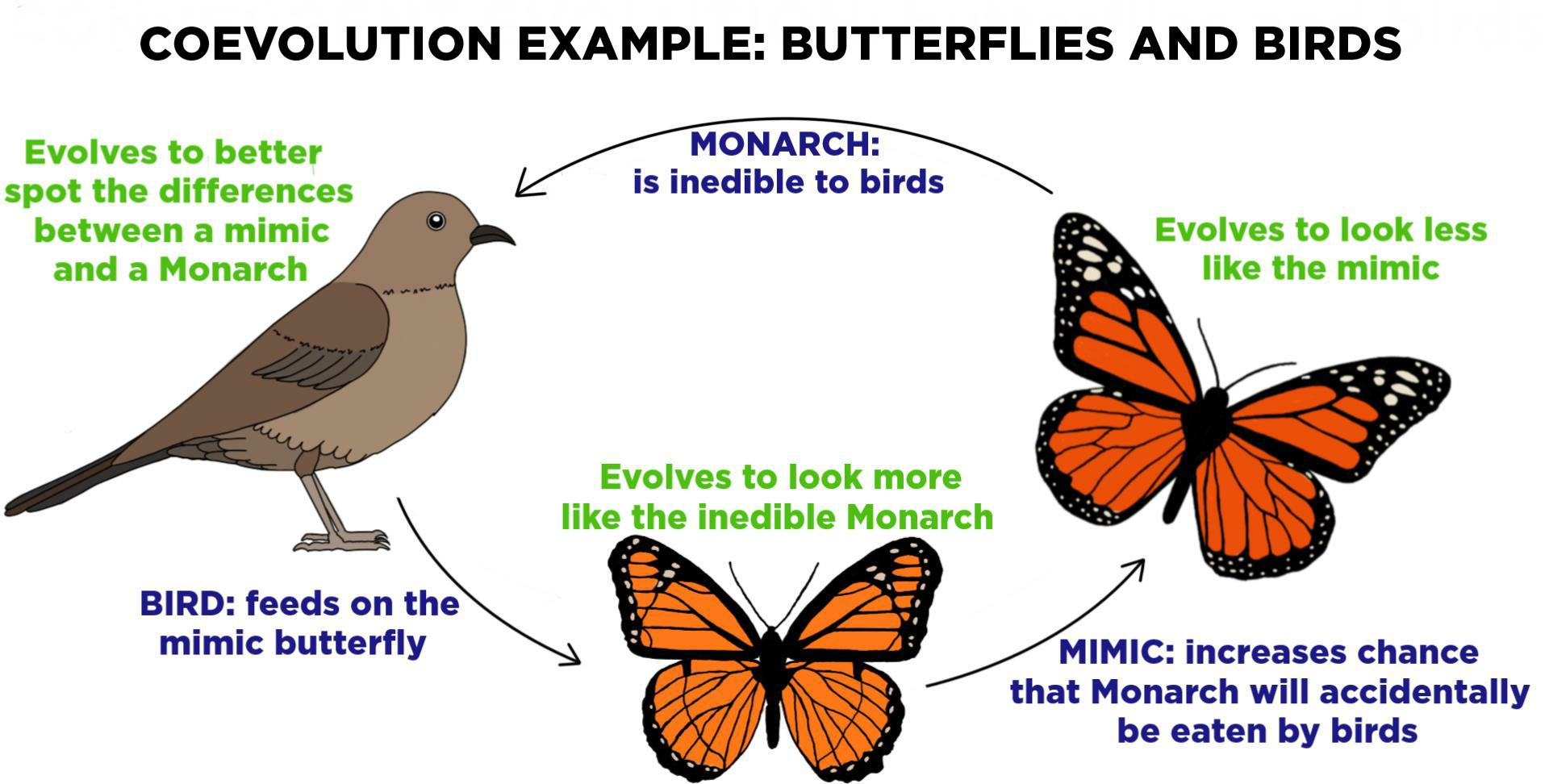
Coevolution two species who interact evolve together
Phylogenetic
Dear King Philip Came Over For Good Soup
Dear → Domain
King → Kingdom
Philip → Phylum
Came → Class
Over → Order
For → Family
Good → Genus
Soup → Species
Skin Color
Darker skin (eumelanin) = more melanin, less Vitamin D, and more folate
Light Skin (pheomelanin) = less melanin, more Vitamin D, and less folate
Brown skin (eumelanin) = mid
Unit 8 - Ecology
Animal Behavior
https://docs.google.com/presentation/d/1E270nqgwD5IppIcbpq3kHZvaQpq4y2dasZK2liVLMTk/edit#slide=id.p1
Behavior is an action carried out by the muscles under control of the nervous system in response to the stimulus
Allows an animal to respond to its environment
Survival
Reprodcution
It’s subjective to natural selection
Those with beneficial behaviors survive better and pass those behaviors on to offspring
Types of Behavior
Innate Behaviors that are present at birth – nearly all individuals in a population exhibit this behavior
Learned Behavior that is modified based on experience
Fixed Action Patterns is the behavior in which a sequence of unlearned acts is directly linked to a specific stimulus (aka reflexes on steroids)
Migration is an example of animals responding to an environmental stimulus
It is a regular, long-distance change in location
Animals are guided by the position of the sun, Earth’s magnetic field and their evolved ancestral behaviors in order to reach a particular destination
Foraging Behaviors are influenced by natural selection
This includes: obtaining and eating food
The optimal foraging model states that natural selection should favor a foraging behavior that minimizes the costs of foraging while maximizing the benefits
The optimal time is defined
Mating Behaviors and Choices
The needs of the young are important for the evolution of mating systems
Species that are young require more care and tend to be monogamous
Species whose young require less care tend to be promiscuous
Certainty of Paternity Males who know that they are the father will protect their offspring, but if they are uncertain then they won’t be as involved
Animal Communication a stimulus is transmitted from one animal to another is called a signal
Transmission and reception of signals constitute animal communication
Communication allows animals to interact with each other
Methods:
Visual - through sight
Tactical
Electrical
Chemical
Bees are able to communicate with one another where food sources are by dancing
Based on dance moves the bees perform for their bee buds, the other members of the hives are able to find where te food is
Allows the needs to respond to their environment
Pheromones animals that communicate through odors or tastes emit a chemical substance
It can be used for:
Find mates
Dissuade predators
Establish dominance
Serve as alarm signals to warn others of danger
Genetic Basis of Behavior
Certain individual genes can have a profound impact on behavior
A single gene can have a profound impact on behavior
Ex: Prairie vole
Prairie Vole Love
Male meadow voles are solitary and do not form lasting relationships with mates
Male prairie voles form a pair bond with a single female after they mate, taking care of their offspring
Male prairie voles release a neurotransmitter known as vasopressin during mating
If prairie voles are treated with a drug that inhibits the vasopressin receptor, they don’t form pair bonds after mating
When the vasopressin receptor gene is inserted into the meadow voles, they take care of their young
Altruism describes behavior that reduces an individual’s fitness but increases the fitness of other individuals in the populations
Allows for the success of the whole, which ends up helping the individual
Having a strong community = safety + security for all members
This occurs in families, as the members of the family have a lot of common DNA
Energy Flow
https://docs.google.com/presentation/d/1MtSXJt8Etf8k_gGL7y5N0YcgxMDY_EPYNhuKiE9yauE/edit#slide=id.p1
 Knowt
Knowt
