Chapter 3: Water and Life
- Water is the only common substance on Earth to exist in the natural environment in all three physical states of matter
- Ratio of ice and to liquid water on earth is changing due to climate change, having different effects on wildlife
- Ex. blooms of plankton are forming in the Arctic due to warmer temperatures, but species depending on arctic ice are suffering
Concept 3.1: Polar covalent bonds in water molecules result in hydrogen bonding
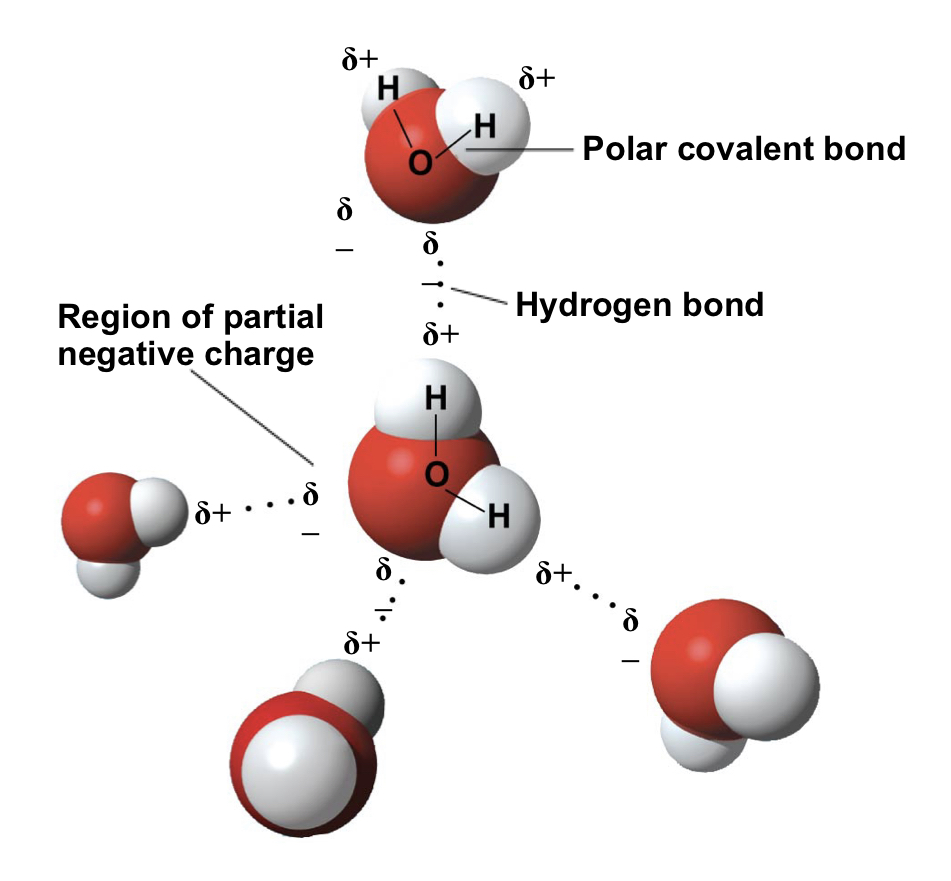
In a water molecule, the electrons of the ==polar covalent bonds== spend more time near the oxygen than the hydrogen atoms
- due to unequal sharing of electrons as oxygen is more electronegative
- forms a bent shape with a 104.5 degree angle
water is a ==polar molecule==, meaning that the overall charge of the molecule is uneven
The oxygen of the water molecule has two regions of partial negative charge, and each hydrogen has a partial positive charge
Atoms in a water molecule are held together with hydrogen bonds
When water is in its liquid form, the hydrogen bonds are fragile
- they are easy to form, break, and re-form with high frequency
The properties of water emerge from this hydrogen bonding
Concept 3.2: Four emergent properties of water contribute to Earth’s suitability for life
4 Elements are:
- cohesive behaviour
- ability to moderate temperature
- expansion upon freezing
- versatility as a solvent — due to polarity
^^Cohesion of Water Molecules^^
- water molecules stay close to each other as a result of hydrogen bonding
- at any given moment, the molecules of liquid water are linked by multiple hydrogen bonds, making water more structured than other liquids
- collectively, hydrogen bonds hold water molecules together, a phenomenon called ==cohesion==
- ==adhesion==: an attraction between substances
- ==cohesion==: an attraction between the same substance
- IN PLANTS: cohesion helps with the transport of water and nutrients against gravity because as the water vapour evaporates from the leaves, hydrogen bonds cause water molecules leaving the leaf veins to tug on molecules further down, and transmits an upward pull through water-conducting cells all the way to the roots
- adhesion also plays a role as the adhesion of water by hydrogen bonds to cell walls helps to counter the downward pull of gravity
- ==surface tension==: a measure of how difficult it is to break the surface of a liquid
- water has an unusually high surface tension due to hydrogen bonding between the molecules at the air-water interface and to the water below
- water hydrogen bonds to itself, not the air around above, causing asymmetry, which is why surface tension is so high
^^Moderation of Temperature by Water^^
- water absorbs heat from warmer air and release stored heat to cooler air
- water can absorb or release a large amount of heat with only a slight change in its own temperature - makes it a good ==heat sink==
^^Temperature and Heat^^
- ==kinetic energy==: the energy of motion
- ==thermal energy==: the kinetic energy associated with random motion of atoms or molecules
- *atoms and molecules always having kinetic energy because they are always moving
- ==temperature==: the average kinetic energy of the molecules in a body of matter
- coffeemaker example
- temperature does not depend on volume, while thermal energy does as it focus on the total kinetic energy, rather than just the average
- even though a coffee pot is at a higher temp. than a swimming pool, the pool still has more thermal energy because it has much greater volume
- ==heat==: the transfer of thermal energy from one body of matter to another
- happen when the objects are brought together
- ==calorie (cal)==: the amount of heat required to raise the temperature of 1g of water by 1ºC
- also the amount of heat released when 1g of water cools by 1ºC
- ==kilocalorie (kcal)==: the amount of heat required to raise the temperature of 1 kg of water by 1ºC
- food labels are actually in kcal
- ==joule (J)==: another unit of energy
- 1 J = 0.239 cal or 1 cal = 4.184 J
^^Water’s High Specific Heat^^
- ==specific heat==: the amount of heat that is must be absorbed or lost by a substance for 1g of that substance to change its temperature by 1ºC
- can be seen as how well a substance resists changing its temperature when it absorbs or releases heat
- the specific heat of water is 1 cal / (g • ºC)
- water resists changing its temperature because of its high specific heat
- water will change its heat less than other materials when it either absorbs or loses a given amount of heat
- water’s high specific heat can be traced to hydrogen bonding
- requires lots of energy to form or break these hydrogen bonds
- the high specific heat of water minimizes temperature fluctuations to within limits that permit life
^^Evaporative Cooling^^
- ==evaporation (vaporization)==: transformation of a substance from liquid to gas
- when molecules of liquids gain enough speed and energy to overcome attractions, they depart from the liquid and become gas
- ==heat of vaporization==: the heat a liquid must absorb for 1g to be converted to gas
- water’s high heat of vaporization is also due to its hydrogen bonds
- ==evaporative cooling==: as a liquid evaporates, its remaining surface cools
- evaporative cooling of water helps to stabilize temperatures in organisms and bodies of water
^^Floating of Ice on Liquid Water^^
- ice floats in liquid water because hydrogen bonds in ice are more “ordered”, making ice less dense than water
- water forms a crystalline lattice structure when it is frozen, causing more order
- water is most dense at 4ºC
- in ponds, the deepest water in 4ºC
- in the winter, the water at the top gets warmed by the sun rays through the ice, then sinks to the bottom and is replaced by warmer water from below, creating a cycle
- if ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth
- many scientists are worried that global warming is having a profound effect on icy environments around the globe
- the rate at which glaciers and Arctic sea ice are disappearing poses an extreme challenge to animals that depend on ice for their survival
^^Water: The Solvent of Life^^
- ==solution==: liquid that is a completely homogeneous mixture of substances
- concentration of substances are even throughout the entire liquid
- ==solvent==: dissolving agent of a solution
- ==solute==: substance that is dissolved in a solution
- ==aqueous solution==: solution in which water is the solvent
- water is a versatile solvent due to its polarity
- ==hydration shell==: a sphere of water molecules surrounding each ion when an ionic compound is dissolved in water
- water can also dissolve compounds made of nonionic polar molecules - ex. sugar
- even large molecules such as proteins can dissolve in water if they have ionic and polar regions
^^Hydrophilic and Hydrophobic Substances^^
- ==hydrophilic==: substance that has an affinity for water (has a slight charge to it)
- ==hydrophobic==: substance that does not have an affinity for water (doesn’t have a charge)
- in some cases, substances can be hydrophilic without actually dissolving
- ex. cotton towel - made up giant cellulose molecules
- oil molecules are hydrophobic because they have relatively non polar bonds
- hydrophobic molecules related to oils are the major ingredients of cell membranes (the phospholipid bilayer — hydrophilic head with hydrophobic tail)
^^Solute Concentration in Aqueous Solutions^^
- most chemical reactions in organisms involve solutes dissolved in water
- when carrying out experiments, we use mass to calculate the number of solute molecules in an aqueous solution
- ==molecular mass==: the sum of all masses of all atoms in a molecule
- ==molarity (M)==: the number of moles of solute per liter of solution
Concept 3.3: Acidic and basic conditions affect living organisms
a hydrogen atom in a hydrogen bond between two water molecules can shift from one to the other
- the hydrogen atom leaves its electron behind and is transferred as a proton, or hydrogen ion (H+)
- the molecule that lost the proton is now a hydroxide ion (OH-)
- the molecule with the extra proton is now a hydronium ion (H30+)
water is in a state of dynamic equilibrium in which water molecules dissociate at the same rate at which they are being reformed
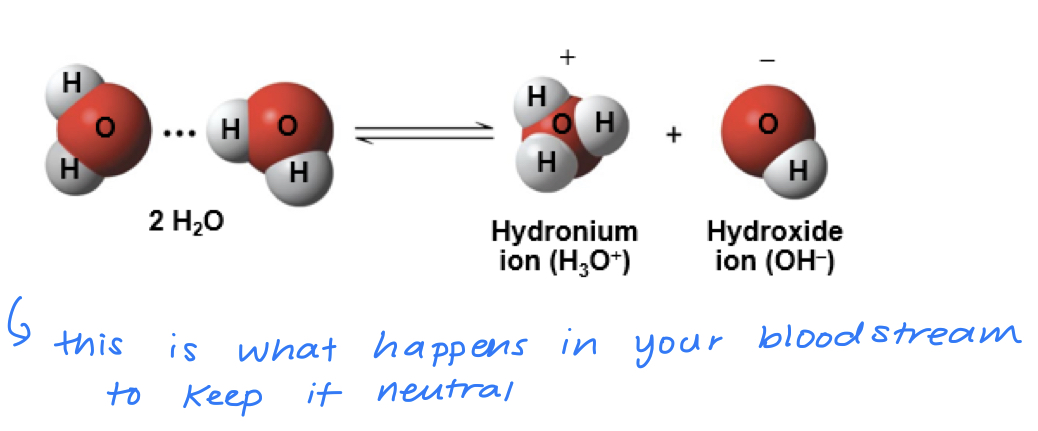
concentration of H+ and OH- are equal in pure water
though statistically rare, the dissociation of water molecules has a great effect on organisms
- changes in concentrations of H+ and OH- can drastically affect the chemistry of a cell
adding acids and bases changes the concentrations of H+ and OH-
pH scale is used to measure whether a solution is acidic or basic
^^Acids and Bases^^
- ==acid==: substance that increases the H+ concentration of a solution
- ==base==: substance that reduces the H+ concentration of a solution (accepts H+ or forms OH-)
- strong acids and bases dissociate completely in water
- weak acids and bases reversibly release and accept back hydrogen ions, but can still shift the balance of H+ and OH- from neutrality
^^The pH Scale^^
- most biological fluids have pH values in the range of 6-8
- most fluids in the body have a certain pH because enzymes working within these fluids require a certain pH to survive and be effective
- ex. amylase needs neutral environment and pepsin needs an acidic environment
- all enzymes are proteins, but not all proteins are enzymes
^^Buffers^^
- the internal pH of most living cells is close to 7
- buffers: substances that minimize changes in concentrations of H+ and OH- in a solution
- carbonic acid is a good buffer of the body — helps keep body neutral
- most buffer solutions contain a weak acid its corresponding base, which combine reversibly with H+ ions