Atomic structure of Atoms.
Hund’s rule - orbitals in the same energy level must have one electron before a second electron can be placed
Aufbau principle - states that electrons start by filling the lowest energy orbitals (n=1) before filling in the highest.
Pauli exclusion principle - only two electrons with opposite spins can occupy any one orbital.
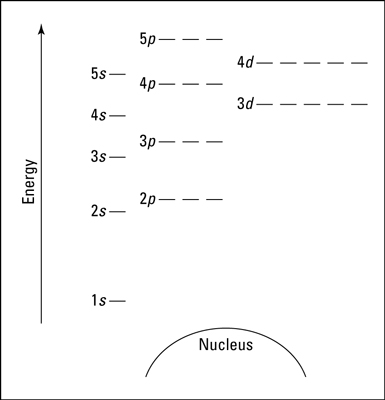
The distance between the atomic shells is not equal - n=1, is further from the rest of the energy levels. The d-sublevel of the previous energy level is slightly above that of the s-sublevel (3d is slightly above 4s)
To find the max number of electrons occupying an energy level - 2n²
To find the number of orbitals in energy level - n²
The highest n value is considered a valence shell
D-sublevel are not valence electrons; only s and p
Quantum Numbers
Principal Quantum number (n) - the principal Quantum number relates to the distance from the nucleus of electrons (energy levels) and their size. They are whole numbers starting from 1 - 1, being the most stable and closest to the nucleus - As the value of n increases, the energy for an electron to occupy orbital increases.
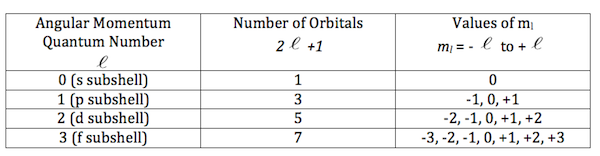
Secondary Quantum number (l)- the secondary Quantum number relates to the shape of the electrons and their sub-levels. They must be whole numbers - including 0 to n-1.
Magnetic Quantum number (ml) - the magnetic quantum number describes the orientation of the electron’s orbit. The values must be from -l. to +l - including 0 - the number of different values of ml equals the number of possible orbitals.
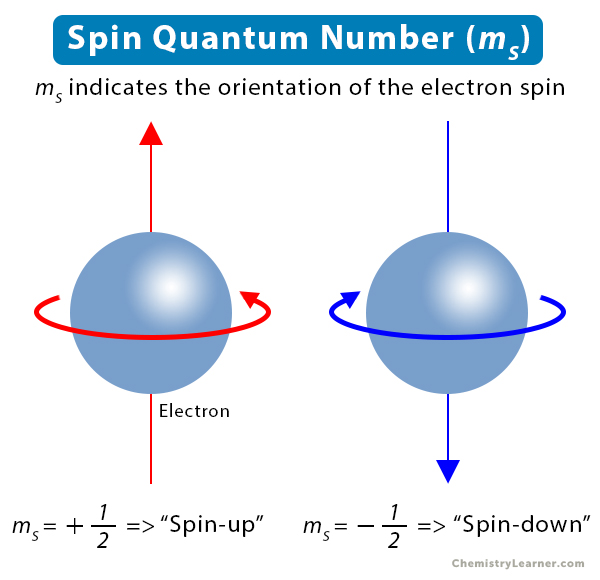
 Spin Quantum number - the spin quantum number describes the angular momentum of an electron, varying from +1/2 or -1/2. According to Pauli's exclusion principle, no two electrons can have the same set of quantum numbers - they may have the same principal, secondary and magnetic, but they differ in the spin quantum number.
Spin Quantum number - the spin quantum number describes the angular momentum of an electron, varying from +1/2 or -1/2. According to Pauli's exclusion principle, no two electrons can have the same set of quantum numbers - they may have the same principal, secondary and magnetic, but they differ in the spin quantum number.
+1/2 - arrow is up
-1/2 - arrow is down
Electron configuration
The location of the electrons in an energy level of an atom or ion is called electron configuration.
As you move from left to right on the periodic table, the electrons of the atoms increase.
Aufbau - “building up” in German - principle states that electrons start by filling the lowest energy orbitals (n=1) before filling in the highest.
Shorthand/ noble gas configuration puts the nearest proceeding noble gas into brackets and continues the electron configuration from there.
Valences & Transition Metals
It is less critical for filling the s-sublevel than the d-sublevel. In transition metals, the s-sublevel would transfer one of its electrons to a half-filled orbital of the d-sublevel. To be at the lowest energy state (most stable state). Half-filled and filled orbitals are at a lower energy than unfilled orbitals.
Ionization Energy & Electron Affinity
Ionization energy
Ionization energy is the energy required to remove an electron in the gas phase.
Increases as you go up and to the right of the periodic table - with exceptions
The bigger the atom, the easier it is to take electrons from it.
The ionization energy increases according to the charge of a gaseous atom/ion (as the charge increases, the ionization energy also increases.
It would take more energy to remove an electron in the s-sublevel than in the p-sublevel - because the s-sublevel is closer to the nucleus.
It is harder to remove an unpaired electron than a paired electron - paired electrons create extra repulsion and instability. Hence, the atom is more adamant to give away that electron of opposite spin to become more stable.
It is easier to remove an electron of a higher energy level than a lower energy level (easier to remove a 3s electron than a 2p electron) - because the electron in the lower energy level is closer to the nucleus.
Removing a ‘core’ electron is harder than a valence electron.
Electron affinity
Electron affinity is the energy released when an atom gains an electron in the gas phase.
Increases as you go up and to the right of the periodic table
As atomic radius decreases, EA increases.
Atoms with a filled octet have very low electron affinity because they do not want/require any more electrons. The following electron must be in a new sublevel (or even energy level), creating instability.
Atoms with half-filled sublevels (np³) would have low EA because the next electron would need to be paired with one of the unpaired electrons, creating extra repulsion and instability.