AP Biology Unit 1 - Chemistry of Life
<<1.1 -<< <<Properties of Water<<
Water is a covalent bond of two hydrogen and one oxygen atom. Oxygen is more electronegative than hydrogen, which gives the hydrogens a partial positive (+) charge, and the oxygen a partial negative (-) charge, making water polar.
Hydrogen Bonds
Hydrogen bonds are weak bond interactions between two molecules. This happens in water due to the polarity of the molecule (positive hydrogen bonds with negative oxygen).
Hydrogen bonds between two of the same molecules are called cohesion. Hydrogen bonds between two different molecules are called adhesion
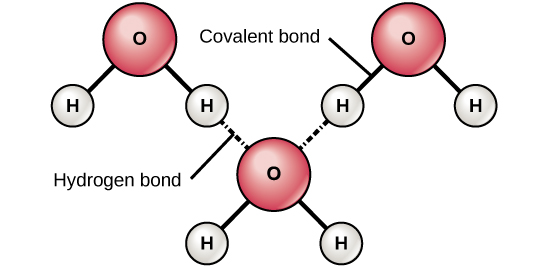 Properties of Water
Properties of Water
Surface tension is the result of increased hydrogen bonds between water molecules at the surface. Aquatic plants rest on the surface of water (due to surface tension) for increased sunlight access.
Water has a high solvency in a liquid state due to its polarity. Water is known as a universal solvent, although it cannot dissolve everything. When other polar substances (like NaCl) dissolve in water, the molecules form a sphere of hydration around each atom. High solvency benefits life as nutrients dissolved in water allow for the nutrients to easily access living cells.
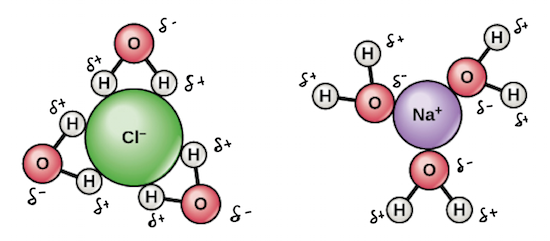
The cohesive properties of water give it a high heat capacity, which allows water to absorb thermal energy and be resistant to sudden temperature changes. Aquatic organisms depend on this water property so they can maintain temperature regulation.
When water freezes, the molecules expand, forming six-sided crystalline structures. Since the molecules expand, this makes solid water less dense than liquid water, allowing ice to float. This allows for life to exist beneath ice in arctic climates.
Capillary action is when liquid water flows into narrow spaces (like a tube) in opposition to gravity. This is due to the adhesive property (molecules bond with the walls of the narrow space) and cohesive property (molecules bond together) of water. Capillary action is how plants can access water in the soil through their roots.
<<1.2 - Elements of life<<
Living systems require a constant input of energy to accomplish processes (growth, reproduction, organization) that are necessary to sustain life. Energy cannot be created/destroyed, yet it can be transformed. Living systems store energy in chemical bonds.
Macromolecules
Living systems require change of matter with the environment, it is necccesary to build molecules. Biological macromolecules are made out of six elements: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorous, and Sulfur.
Proteins always contain C,H,O and N. They may sometimes contain S, however they never contain P. Carbohydrates always contain C, H, and O, and never contain N, P, or S. Nucleic acids always contain C, H, O, N, and P, but never contain S. Lipids always have C and H, and sometimes may have N, (very little) O, P (in case of phospholipids), or S.
Carbon
Carbon bonds with other carbon atoms to create carb skeletons. Other atoms attach to create complex macromolecules. They can bond in chains, rings, and branches. Carbon-containing molecules store energy, and form cell structures.
<<1.3 - Biological Macromolecules<<
Monomers are chemical subunits that create polymers. Polymers are macromolecules that are made up of many monomers. Covalent bonds are formed between two monomers to create polymers. Monomers have specific chemical properties that allow them to interact with one another.
Dehydration Synthesis & Hydrolosis
Dehydration synthesis is a reaction that creates macromolecules, by removing H and OH from two interracting monomers and forming a covalent bond between the two monomers. The H and OH combine to form water as a byproduct of the reaction.
Hydrolosis is the opposite of dehydration synthesis. In a hydrolysis reaction, polymers are hydrolyzed (broken down) into separate monomers. The covalent bonds between them are cleaved (broken). Water molecules also hydrolyze and their components (H and OH) are added to monomers.
These processes happen because when macromolecules are consumed by the body, they need to be broken down in order to be absorbed.
<<1.4 - Properties of Biological Macromolecules<<
Function and Structure
The properties of molecules are determined by their structure and function. A change in the structure results in the change of the function of a macromolecule.
Nucleic Acids (DNA/RNA)
The basic structure of a nucleic acid contains a 5-carbon sugar, 1-3 phosphate groups, and a nitrogenous base. There is a difference in sugar between DNA and RNA (deoxyribose in DNA vs ribose in RNA), as well as a difference between the nitrogenous bases (both can contain Adenine, Guanine, and Cytosine, but DNA contains Thymine while RNA contains Uracil).
Proteins (Amino Acids)
Amino acids have directionality; they contain an amino terminus (typicaly NH2) and a carboxyl (COOH) terminus. R groups attached below the central carbon determine the properties of amino acids (polar amino acids will have a highly electronegative atom attached to them (like O), and non polar ones with have a series of hydrocarbons (CH). Large proteins have many different amino acids, which result in regional differences in the protein.
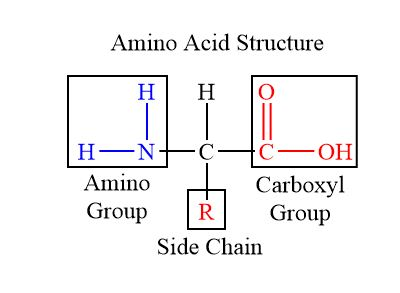
Carbohydrates and Lipids
Specific monomers of carbohydrates determine the properties and functions of the carb.
Lipids consist of fatty acid chains that determine structure and function depending on their saturation. Phospholipids have hydrophobic (water-fearing) and hydrophilic (water-loving) regions that interact with other molecules. Phospholipids and specific proteins make up membranes. Hydrophillic regions of the membranes can interact with each other and the water environment that surrounds them, while hydrophobic regions can only interact with one another.
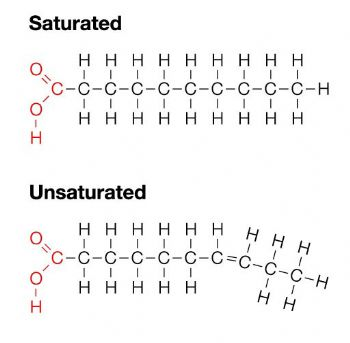
<<1.5 - Structure and Functions of Macromolecule<<
Directionality is he way that a certain molecule is synthesized and is predicated on the asymmetry of the individual monomers.
Nucleic Acid Directionality
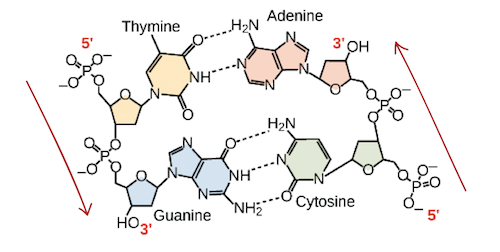
Directionality of a nucleic acid influences the structure of DNA. The linear sequence is characterized by a 3’ hydroxyl and 5’ phosphate of the sugar in the nucleotide. DNA has two antiparallel strands - one side goes 3’ to 5’, the other vice versa. Ademine - Thymine bases have two hydrogen bonds holding them together, while Guanine - Cytosine bases have three hydrogen bonds. Hydrogen bonds between the pairs stabilize strands of DNA. During synthesis of a DNA strand, nucleic acids can only be added to the 3’ end of a strand.
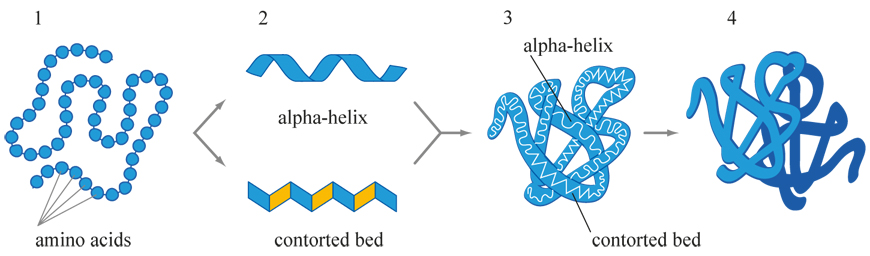
Protein Directionality
Proteins are comprised of long chains of amino acids (called polypeptide chains) with amino and carboxyl termini. Amino acids connect at the carboxyl terminus by covalent bonds (called peptide bonds). There are four structures of proteins:
Carbohydrate Directionality
Carbs are comprised of linear chains of sugar monomers. The smallest of directional changes can result in drastic function differences. Carbohydrate polymers can be in linear formations or in branches.
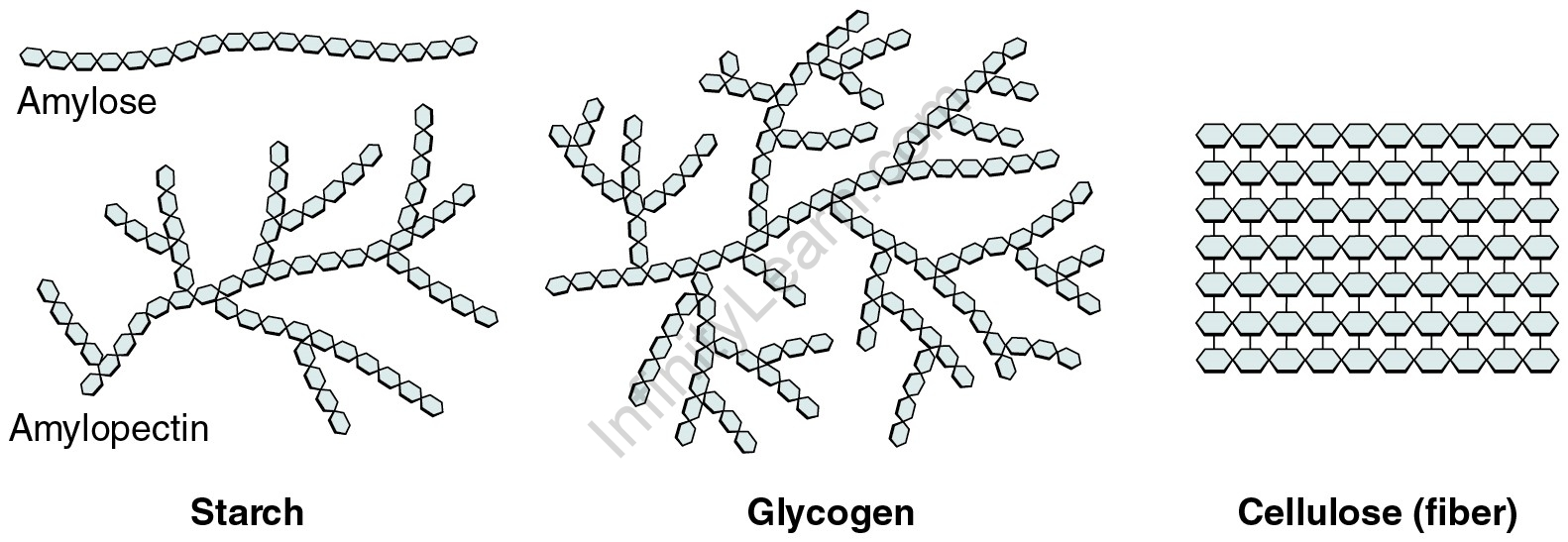
Starch and glycogen function as energy storage, while cellulose functions as support to the cell.