Chapter 16: Reaction Energy Study Guide
Section 1: Thermochemistry
Define Thermochemistry
Know the differences between temperature and heat
Define Calorimeter
Be able to use the temperature change and known mass of a rxn to determine the amount of energy released or absorbed
Define temperature
Relationship between the amount of kinetic energy and temperature in a reaction
what does this mean for what the measurement of temperature is based on?
Define heat of reaction
Phase change = change in potential energy, whereas heat change= change in kinetic energy
Define Heat
How energy transferred as heat is moved
Know what the quantity of energy transferred as heat during aa temperature change depends on
Know what differences in energy absorption in metals depends on
Define specific heat
Know what pressure conditions specific heat is measured under
know how and what types of problems to use cp=q/m x delta T
know how and what types of problems to use q=cp x m x delta T
what happens if a mixture of hydrogen and oxygen is ignited
understand the energy changes involved in the reaction, including the release of heat and the formation of water vapor.
understand what type of reaction this is (endothermic or exothermic)
define thermochemical equations
be able to identify thermochemical equations
know the rules to writing thermochemical equations
define enthalpy change
define the enthalpy of reaction
know application of the formula: delta H = Hproducts- Hreactants
what happens to the enthalpy of products in an endothermic reaction? what about exothermic reactions?
Is the decomposition of water vapor endothermic or exothermic? why?
Why is it important for the physical states of reactants and products to be included in thermochemical equations?
When is delta H negative? When is it positive?
What are the 4 things to consider when looking at all thermochemical equations?
Understand potential energy diagrams:
be able to determine if its endothermic or exothermic
what represents the potential energy of the reactants?
what represents the potential energy of the products?
What represents the heat of reaction delta H?
What represents the activation energy of the forward reaction?
What represents the activation energy of the reverse reaction?
What represents the potential energy of the activated complex?
Determine if the reverse reaction is endothermic or exothermic.
What points in the diagram would change if a catalyst were added?
Be able to use the temperature change formula: Q= mass x delta T x specific heat capacity
Test questions will ask how many joules are
Be able to use the phase change formula: Q= mass x heat of fusion or Q= mass x heat of vaporization depending on the type of phase change occurring.
516-518: Vonese
Driving Force of Reactions
The change in energy of a reaction system is one of the two factors that allow chemists to predict whether a reaction will occur spontaneously and to explain how it occurs
The randomness of the particles in a system is the second factor affecting whether a reaction will occur spontaneously
Reactions generally move to a lower-energy state.
The majority of chemical reactions in nature are exothermic, resulting in products that are more stable and have lower energy than the reactants.
Reactions naturally tend towards a lower energy state
Some endothermic reactions can occur spontaneously, indicating that energy (such as continued heating) is not the only factor determining spontaneity
Entropy measures randomness in a system.
A naturally occurring endothermic process is melting.
Such as an ice cube melting spontaneously at room temperature as energy is transferred from the warm air to the ice
During melting, the well-ordered arrangement of water molecules in the ice crystal is lost, and a less-ordered liquid phase of higher energy content is formed
A system that can go from one state to another without a decrease in enthalpy does so with an increase in entropy
There is a general tendency in nature to proceed in a direction that increases the randomness of a system
A random system lacks a regular arrangement of its parts. Tendency toward randomness is called entropy.
Entropy, S, can be defined in a simple qualitative way as a measure of the degree of randomness of the particles, such as molecules, in a system.
Entropy in states of matter
Solids: particles in fixed position, vibrating; randomness is LOW, so entropy is LOW
Liquids: particles are moving rapidly and are much farther apart; MORE random, entropy is HIGHER in liquids (compared to solids)
Gases: particles are moving rapidly and are much farther apart; MUCH MORE random, entropy is HIGHER in gases
General rule: Entropy in gases>>liquids>solids
(exception of liquid mercury, which is less than some solids)
The entropy of a pure crystalline solid at absolute zero is zero
As energy is added, randomness of molecular motion increases
Measures of energy absorbed and calculations that are used to determine absolute entropy (standard molar energy) are kJ/(mol • K)
Entropy change, which can also be measured, is defined as the difference between the entropy of the products and the reactants.
An increase in entropy is + ΔS.
A decrease in entropy is - ΔS.
The process of forming a solution almost always involves an increase in entropy because there is an increase in randomness.
This applies to mixing gases, dissolving a liquid in another liquid, and dissolving a solid in a liquid
Example: Sugar in tea
Initial: solid sugar has low entropy as molecules are in one region, separate from water molecules
Then: after dissolving, sugar molecules are thoroughly mixed throughout tea; both sugar and water can be found anywhere, increasing system’s randomness and + ΔS
Free energy changes determine if a reaction is endothermic or exothermic.
Processes in nature are driven towards two directions: towards least enthalpy and greatest entropy
The direction that LOWERS free energy of a system will be the direction natural processes proceed in
Enthalpy and Entropy fight for dominance. The dominant factor determines if forward or reverse reaction is favored
Free energy: the combined enthalpy-entropy function, also known as “Gibbs free energy”
Assess the tendency of enthalpy and entropy to change
Free-energy change: denoted by ΔG; defined as the difference between the change in ΔH and TΔS
Typical Measurements for this equation
TΔS: kj or J
ΔH: kj or J
ΔG: kj or J
ΔS: kj/K
There are 4 possible combinations of terms with this formula
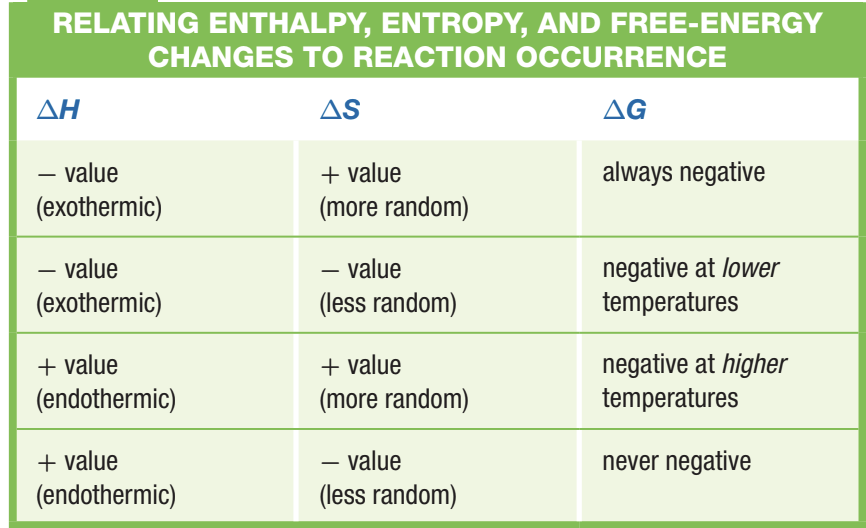
Ex1. Exothermic Reaction
Within this rxn, entropy decreases as we go from 2 moles to 1 mol of gas
Since ΔH is negative, this rxn is exothermic
The enthalpy term predominates in this rxn
Ex2. Endothermic Reaction
Even though the entropy increase would normally push the forward reaction to occur spontaneously at room temperature, the positive ΔG tells us otherwise
Since ΔH is positive, this rxn is endothermic
Thermochemistry
501-508: Sage
Thermochemistry- is the study of the transfers of energy as heat that accompany chemical reactions and physical changes
Temperature and heat are related but not identical
Calorimeter- the energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter
Energy given off (or absorbed) during the reaction is equal to the energy absorbed (or given off) by the known quantity of water.
Amount of energy is determined from the temperature change of the known mass of surrounding water
Data collected from calorimetry experiments are temperature changes because energy cannot be measured directly; but temperature, is directly measurable
Temperature- is a measure of the average kinetic energy of the particles in a sample of matter.
The greater the kinetic energy of the particles in a sample, the higher the temperature is and the hotter it feels
To assign numerical values to temperature, it is necessary to define a temperature scale.
For calculations in thermochemistry, we use the Celsius and Kelvin scales.
Celsius and Kelvin temperatures are related by the following equation
K= 273.15 + oC
The ability to measure temperature is thus based on energy transfer; measured in joules
A joule is the SI unit of heat as well as all other forms of energy
N x m = kg x m2s2
Heat can be thought of as the energy transferred between samples of matter because of a difference in their temperatures
Energy transferred as heat always moves spontaneously from matter at a higher temperature to matter at a lower temperature
Energy transfer varies from reaction to reaction
The quantity of energy transferred as heat during a temperature change depends on the nature of the material changing temperature, the mass of the material changing temperature, and the size of the temperature change
EX: one gram of iron heated to 100.0oC and cooled to 50.0oC in a calorimeter transfers 22.5 J of energy to the surrounding water.
But one gram of silver transfers 11.8 J of energy under the same conditions.
Difference depends on the metals’ differing capacities for absorbing this energy.
Specific heat is the amount of energy required to raise the temperature of one gram of a substance by one Celsius degree (1oC) or one kelvin (1 K) (because the sizes of the degree divisions on both scales are equal)
Values of specific heat can be given in units of joules per gram per Celsius degree, J/(g x oC), joules per gram per kelvin, J/(g x K), or calories per gram per Celsius degree, cal/(g x oC)
Specific heat is measured under constant pressure conditions, so its symbol, cp, has a subscripted p as a reminder. In the equation, cp is the specific heat at a given pressure, q is the energy lost or gained, m is the mass of the sample, and T represents the change in temperature
cp=qm x T
This equation can be rearranged to give an equation that can be used to find the quantity of energy gained or lost with a change in temperature
Energy Lost or Gained q=cp m T
Heat energy is transferred during a reaction
Energy absorbed as heat during a chemical reaction at constant pressure is represented by H. The H is the symbol for quantity called enthalpy. It is not practical to talk about enthalpy as a quantity, because we have no way to directly measure the enthalpy of a system
Only changes in enthalpy can be measured. The Greek letter (“delta”) stands for “change in.”
Therefore, H is read as “change in enthalpy”.
An enthalpy change is the amount of energy absorbed by a system as heat during a process at constant pressure H = Hproducts- Hreactants
The enthalpy of reaction is the quantity of energy transferred as heat during a chemical reaction. (HEAT OF REACTION)
Enthalpy of Reaction in Exothermic Reactions
If a mixture of hydrogen and oxygen is ignited, water will form and energy will be released explosively
Energy that is released comes from the reactants as they form products
Because energy is released, the reaction is exothermic, and the energy of the product, water, must be less than the energy of the reactants.
EX: 2 mol of hydrogen gas at room temp are burned, 1 mol of oxygen gas is consumed and 2 mol of water vapor are formed
2H2(g) + O2(g) —> 2H2O(g)
Experiments have shown that 483.6 kJ of energy are evolved when 2 mol of gaseous water are formed from its elements at 298.15 K.
Modifying the chemical equation to show the amount of energy as heat released during the reaction gives the following expression.
2H2(g) + O2(g) —> 2H2O(g) + 483.6 kJ
This expression is an example of a thermochemical equation, an equation that includes the quantity of energy released or absorbed as heat during the reaction as written
In any thermochemical equation, we must always interpret the coefficients as numbers of moles and never as numbers of molecules.
The quantity of energy released as heat in this or any other reaction depends on the amount of reactants and products
The quantity of energy as heat released during the formation of water from H2 and O2 is proportional to the quantity of water formed; require twice as many moles of reactants and would release 2x more energy as heat
4H2(g) + 2O2(g) —> 4H2O(g) + 967.2 kJ
Producing one-half as much water requires one-half as many moles of reactants and releases only one-half as much energy, or ½ x 483.6 kJ.
H2(g) + ½ O2(g) —> H2O(g) + 241.8 kJ
Enthalpy of Reaction in Endothermic Reactions
The situation is reversed in an endothermic reaction– products have a larger enthalpy than reactants.
Decomposition of water vapor is endothermic; it is the reverse of the reaction that forms water vapor
In endothermic reactions, enthalpy now appears on the reactant side of the thermochemical equation but no changed value
2H2,O(g) + 483.6 kJ —> 2H2(g) + O2(g)
The physical states of reactants and products must always be included in thermochemical equations because they influence the overall amount of energy as heat gained or lost.
EX: energy need to decompose water would be greater than 483.6 kJ if we started with ice, becauze extra energy would be needed to go from ice to liquid and then to vapor
Thermochemical Equations
Thermochemical equations are usually written by designating the value of H
For exothermic reaction, H is always negative because the system loses energy.
2H2(g) + O2(g) —> 2H2O(g) H= -483.6kJ
For endothermic reaction H is always positive because the system gains energy.
2H2O(g) —-> 2H2(g) + O2(g) H= +483.6kJ
Since energy as heat is absorbed, the enthalpy of the reactants is lower than the final enthalpy of the products, and H is positive.
When looking at all the thermochemical equations, consider the following.
The coefficients in a balanced thermochemical equation represent the number of moles of reactants and products and never the number of molecules. They can be fractions when necessary
The Physical state of the product or reactant involved in a reaction is an important factor, and, therefore, must be included in the thermochemical equation
The change in Enthalpy represented by a thermochemical equation is directly proportional to the number of moles of substances undergoing a change. If 2 mol of water are decomposed, twice as much enthalpy
The value of the enthalpy change, H, is usually not significantly influenced by changing temperature
Enthalpy of formation is the energy change when elements form one mole of a compound
Thermochemical data are often recorded as the enthalpies of such composition reactions.
The molar enthalpy of formation is the enthalpy change that occurs when one moles of a compound is formed from its elements in their standard state at 250C and 1 atm.
Enthalpies of formation are given for the standard states of reactants and products; usually atmospheric pressure and room temp 298.15K
To signify that a value represents measurements on substances in their standard states, a o sign is added to the enthalpy symbol, giving delta H for the standard enthalpy of a reaction.
Adding a subscript f, as in Hfo further indicates a standard enthalpy of formation.
508-514 (start from “exothermic compounds tend to be very stable”): Pham
Exothermic compounds tend to be very stable
A compound with a large negative enthalpy of formation releases a large amount of energy as heat when it’s formed → stable
Elements in standard states have ∆H0f = 0
The majority of enthalpies of formation are negative
∆H0f of CO2 is -393.5 kJ/mol therefore, it is more stable than the elements from which it was formed
Compounds with relatively positive or slightly negative values are unstable
Ex: HI is a colorless gas that decomposes at room temperature
Has an enthalpy of formation of +26.5 kJ/mol
As it decomposes, the violet iodine vapor, I2, becomes visible through the container
Compounds with a high positive enthalpy of formation are very unstable and may react or decompose violently
Ex#1: C2H2 reacts violently with oxygen and must be stored in cylinders as a solution in acetone
Ex#2: HgC2N2O2 has a very large enthalpy of formation of +270 kJ/mol which makes it useful as a detonator for explosives
Enthalpy changes in combustion
Combustion reactions produce energy in the form of light and heat when a substance is combined with oxygen
Enthalpy of combustion: the enthalpy change that occurs during the complete combustion of one mole of a substance
Enthalpy of combustion is defined in terms of one mole of reactant, and enthalpy of formation is defined in terms of one mole of product
All substances are in their standard states
General enthalpy notation, ∆H, applies to enthalpies of reaction
∆Hc refers to the enthalpy of combustion
A combustion calorimeter is a common instrument used to determine enthalpies of combustion
A similar apparatus under constant pressure is used to obtain enthalpy measurements
Change in enthalpy is calculated using Hess’s Law
Thermochemical equations can be rearranged and added to give enthalpy changes for reactions not included in the data tables
Hess’s law: the overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process
Energy difference between reactants and products is independent of the route
Measured enthalpies of reaction can be combined to calculate enthalpies of reaction that are difficult or impossible to actually measure
Calculate the enthalpy of formation of methane gas from its elements, hydrogen gas and solid carbon (graphite), at 298.15 K:
In order to calculate the change in enthalpy, we use the combustion reactions of each element
Principles for combining thermochemical equations:
If a reaction is reversed, the sign of ∆H is also reversed
Multiply the coefficients of the known equations so that, when added together, they give the desired thermochemical equation. Multiply ∆H by the same factor as the corresponding equation.
In this case, since methane is on the right side of the thermochemical equation, we must reverse the combustion equation of methane and change the sign of ∆H. This will turn the reaction to an endothermic one.
Since we now have 2 moles of water as a reactant, we will need 2 moles of water as a product
For the combustion of hydrogen, it only produces one mole of water so we would need to multiply everything by 2
Now add the three equations together
Hess’s law states that the enthalpy difference between reactants and products is independent of the pathway
Any enthalpy of reaction may be calculated using enthalpies of formation for all the substances in the reaction of interest, without knowing anything else about how the reaction occurs
Enthalpy of formation is the sum of its sub-reaction enthalpies
When carbon is burned in a limited supply of oxygen, CO is produced
Carbon is first oxidized to CO2 then part of it is reduced with carbon to give some CO
These two reactions occur simultaneously so we get a mixture of CO and CO2
It’s not possible to directly measure the enthalpy of formation of CO(g_ from C(s) and O2(g)
However, we do know the enthalpy of formation of CO2 and enthalpy of combustion of CO
Reverse second equation because we need CO as a product
This diagram is a model for a reaction that takes place in two steps
If we plot the reactions based on their relative energy, you can see the relationship among the values for the enthalpy of formation of CCO
The formation of CO2 is at a level of -393.5 kJ/mol
It shows the reverse of the combustion reaction (+293.0 kJ/mol) is added to that level
The value of the formation of CO is -110.5 kJ/mol.