
Carboxylic acids
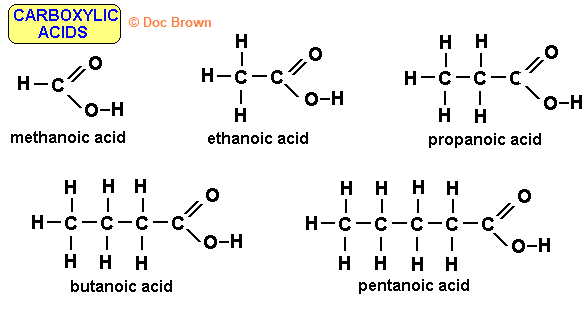
All have -COOH functional group attached to the first carbon
Their general formula is CnH2n+1COOH
They dissolve in water and therefore are weakly acidic solutions
The 1st two are liquid @ r.t.p. (methanoic acid: nettle and ant stings, ethanoic acid: acid found in vinegar)
The suffix is -anoic acid
number of carbons | prefix |
|---|---|
1 | meth- |
2 | eth- |
3 | prop- |
4 | but- |
Due to being weak, organic acids, they undergo the usual acid reactions.
1) acid + alkali → metal salt + water
ethanoic acid + sodium hydroxide → sodium ethanoate + water
CH3COOH + NaOH → CH3COONa + H2O
* note: the salt always ends in “-oate” (e.g. methanoic acid → methanoate)
2) acid + metal carbonate → metal salt + carbon dioxide + water
ethanoic acid + calcium carbonate → calcium ethanoate + carbon dioxide + water
CH3COOH + CaCO3 → (CH3COO)2Ca + CO2 + H2O
*this reaction occurs in the “descaling” of hard water/limescale
Carboxylic acids
All have -COOH functional group attached to the first carbon
Their general formula is CnH2n+1COOH
They dissolve in water and therefore are weakly acidic solutions
The 1st two are liquid @ r.t.p. (methanoic acid: nettle and ant stings, ethanoic acid: acid found in vinegar)
The suffix is -anoic acid
number of carbons | prefix |
|---|---|
1 | meth- |
2 | eth- |
3 | prop- |
4 | but- |
Due to being weak, organic acids, they undergo the usual acid reactions.
1) acid + alkali → metal salt + water
ethanoic acid + sodium hydroxide → sodium ethanoate + water
CH3COOH + NaOH → CH3COONa + H2O
* note: the salt always ends in “-oate” (e.g. methanoic acid → methanoate)
2) acid + metal carbonate → metal salt + carbon dioxide + water
ethanoic acid + calcium carbonate → calcium ethanoate + carbon dioxide + water
CH3COOH + CaCO3 → (CH3COO)2Ca + CO2 + H2O
*this reaction occurs in the “descaling” of hard water/limescale

 Knowt
Knowt
