Lecture_Feb_10_12
Lecture Information
Course:
CHM-107-01
Date:
Feb 10, 12
Instructor:
Dr. K. G. Karaisz
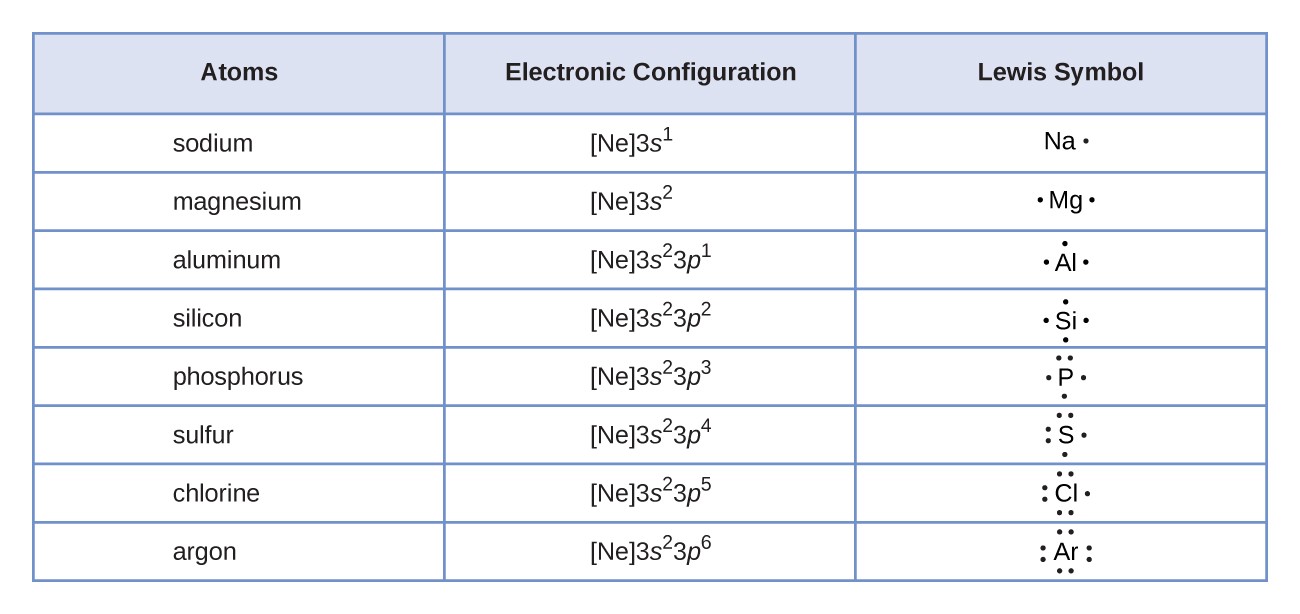
General Guidelines for Writing Lewis Formulas of Molecules
Steps to Follow
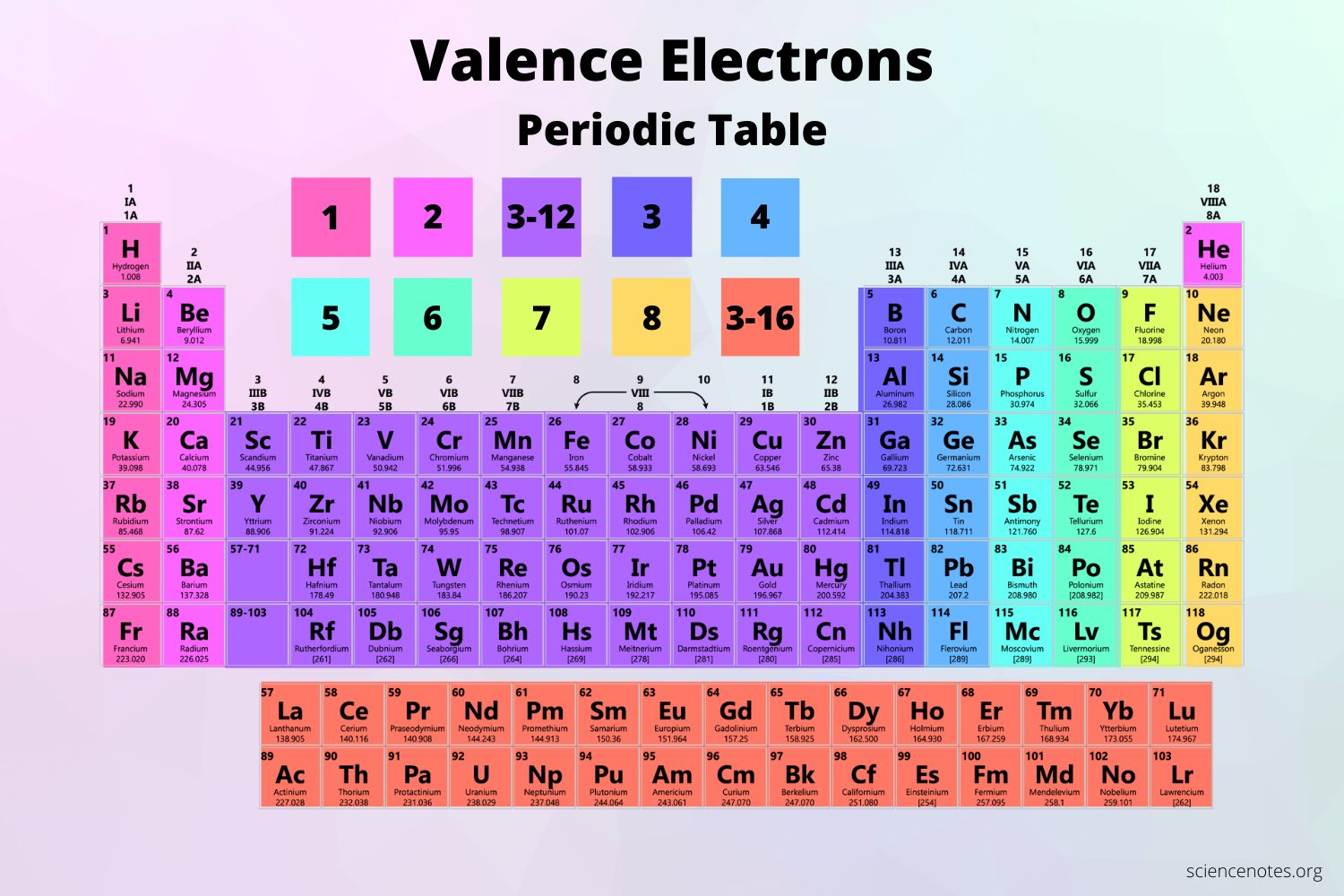
Determine Total Valence Electrons
Count the valence electrons for all elements in the compound, using the periodic table to identify the group of each element, which indicates its valence electron count.
Draw the Skeleton Structure
Typically, the less electronegative element is chosen as the central atom in the structure.
Common central atoms include Carbon (C), Nitrogen (N), and Phosphorus (P), while Hydrogen (H) and Halogens (F, Cl, Br, I) are generally found at the ends of the structure.
Arrange Bonds and Non-Bonding Electrons
Ensure that the structure satisfies both the Octet Rule, where each atom aims for a total of 8 electrons in its outer shell, and the Formal Charge, aiming for a formal charge of zero or as close as possible for stability.
Note that Hydrogen requires only 2 electrons in total to achieve stability.
Example: CH4 (Methane)
Carbon (C): 4 valence electrons (1 atom x 4)
Hydrogen (H): 1 valence electron (4 atoms x 1)
Total valence electrons: 4 (from C) + 4 (from 4 H) = 8 electrons
Structure: H | H-C-H | H
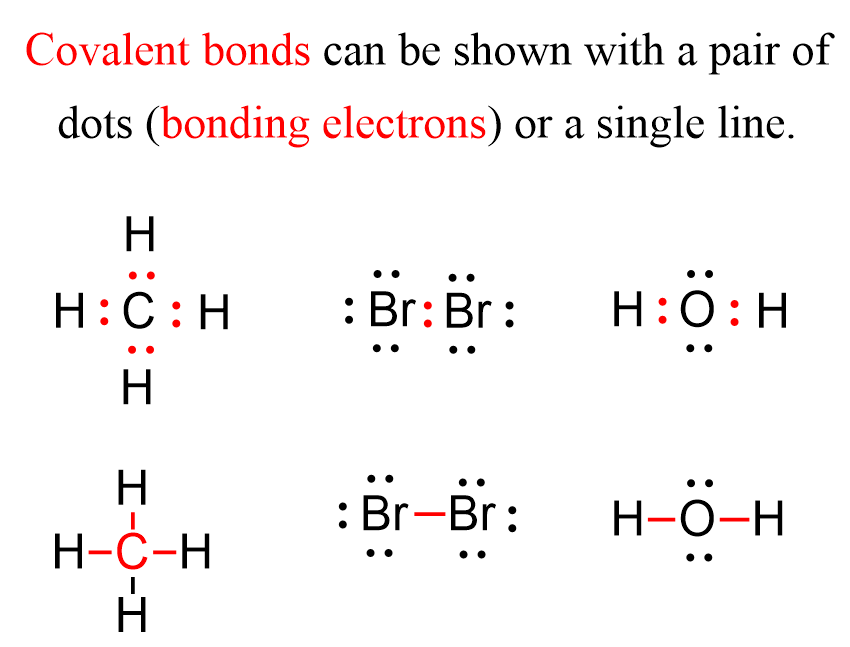
Examples of Lewis Structures
Example CO2
Valence Electrons:
Carbon (C): 4 electrons (1 x 4)
Oxygen (O): 6 electrons (2 x 6)
Total Valence Electrons: 4 + 12 = 16 electrons
Structure: O=C=O (Corrected for Octet Rule)
Example NCl3
Valence Electrons:
Nitrogen (N): 5 electrons (1 x 5)
Chlorine (Cl): 7 electrons (3 x 7)
Total Valence Electrons: 5 + 21 = 26 electrons
Structure: Cl-N(Cl)-Cl, satisfying the Octet Rule for each atom.
Example C2H3Br
Valence Electrons:
Carbon (C): 4 electrons (2 x 4) = 8 electrons
Hydrogen (H): 1 electron (3 x 1) = 3 electrons
Bromine (Br): 7 electrons (1 x 7) = 7 electrons
Total Valence Electrons: 8 + 3 + 7 = 18 electrons
Diagram should include corrected formal charges for stability.
Shapes (Geometry) of Molecules
Linear Geometry
Examples:
H2: H – H
Br2: Br — Br
O2: O = O
N2: N ≡ N
H – Cl
Bent Geometry
Example: H2O
Configuration:H|OAngle: 105°
Tetrahedral Geometry
Example: Methane (CH4)
Angle: 109.5°
Polarity/Non-Polarity of Molecules
Example H2O:
Geometry is bent, leading to molecular polarity due to the difference in electronegativity between oxygen and hydrogen.
The H-O bond is polar covalent with an EN difference of 1.4.
Resulting in partial charges: δ- on oxygen, and δ+ on hydrogen.
Example CCl4:
Tetrahedral geometry promotes symmetry.
Individual polar covalent bonds (C-Cl) cancel out, making CCl4 a non-polar molecule.
Chemical Naming and Formula Writing
Chemical Naming Conventions
Common Names:
Often lacking formal naming rules and based on historical usage.
IUPAC Naming:
Example: NaCl
IUPAC Name: Sodium Chloride
Common Name: Table Salt
IUPAC Naming Rules
Ionic Compounds:
Name the positive ion first followed by the negative ion (e.g., KBr - Potassium Bromide).
Covalent Compounds:
The element with the lower electronegativity is listed first (e.g., CO2 - Carbon Dioxide).
Binary Compounds:
The second element's name changes to use the suffix “-ide” (e.g., bromine becomes bromide).
Oxidation Numbers:
Represent the combining capacity of an element, which can be positive or negative. Understanding these numbers is crucial in predicting how elements will interact in chemical reactions.
Common Group/Oxidation Number Relationships
Group IA: +1
Group IIA: +2
Group IIIA: +3
Group IVA: +4 or -4
Group VA: -3
Group VIA: -2
Group VIIA: -1
Group VIIIA: 0
Metals with Fixed Oxidation Numbers
Typical matches for metals in Groups IA, IIA, and IIIA with non-metals from Groups VA, VIA, and VIIA.
Example: Na+ and Br- = NaBr.
Example: Mg2+ and Cl- = MgCl2.
Other Examples of Oxidation Numbers
Sodium Oxide: Na2O
Aluminum Oxide: Al2O3
Magnesium Sulfide: MgS
Elements and Periodic Table Overview
Detailed information about each element, including Atomic Number, Atomic Weight, and Electrons per Shell.
The periodic table is organized into groups, emphasizing oxidation states and periodic trends which