CHEMISTRY: ATOMS
Early studies of atoms
Dalton’s Atomic Theory
Dalton theorized that the basic unit of matter is a tiny particle called an atom
Below is a summary
All elements are composed of indivisible atoms
All atoms of a given element are identical
Atoms of different elements are different; they have different masses
Compounds are formed by the combination of atoms of different elements
J.J Thomson’s Electrons
Using a cathode ray tube j.j. Thomson showed smaller units that make up an atom.
Thomson theorized an atom contains small negatively charged particles, which he called electrons
Plum pudding model
In this model, the mass of the rest of the atom was evenly distributed and positively charged, taking up all of the space not occupied by the electrons
Gold Foil Experiment
Ernest Rutherford’s gold foil experiment concluded atoms have a dense central core called a nucleus.
The wave mechanical model
This model represents the electron as having not only properties of mass but also wave-like properties, picturing the atom as having a dense, positively charged nucleus as proposed in the planetary model.
A major difference this model has compared to Bohr's model, is that the electrons pictured in this model are moving in areas called orbitals.
Orbitals: is described as a region in which an electron of a particular amount of energy is most likely to be located.
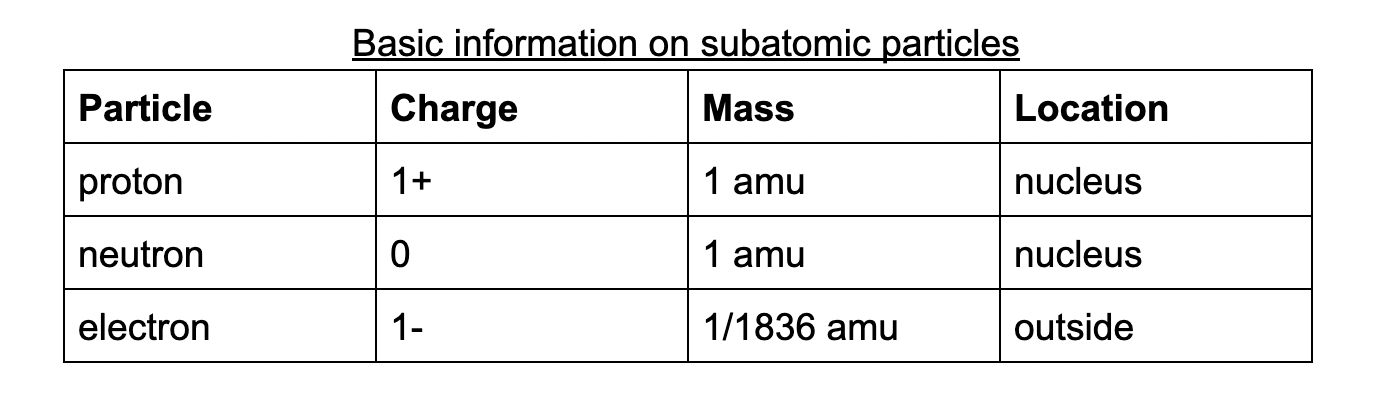
Subatomic particles: particles that compose of an atom
Protons: positively charged particles.
Each atom of a specific element must contain the same number of protons as every other atom of that element. The number of protons in the nucleus of an atom is the atomic number of that element.
Ex. Chlorine has an atomic number of 17. Each chlorine atom contains 17 protons in its nucleus.
Electrons: negatively charged particles.
Electrons occupy the space of an atom outside the nucleus and have a charge equal to the opposite of a proton.
Electrons are less massive than protons and neutrons
Neutrons: neutral particles.
Has approximately the same mass as a proton
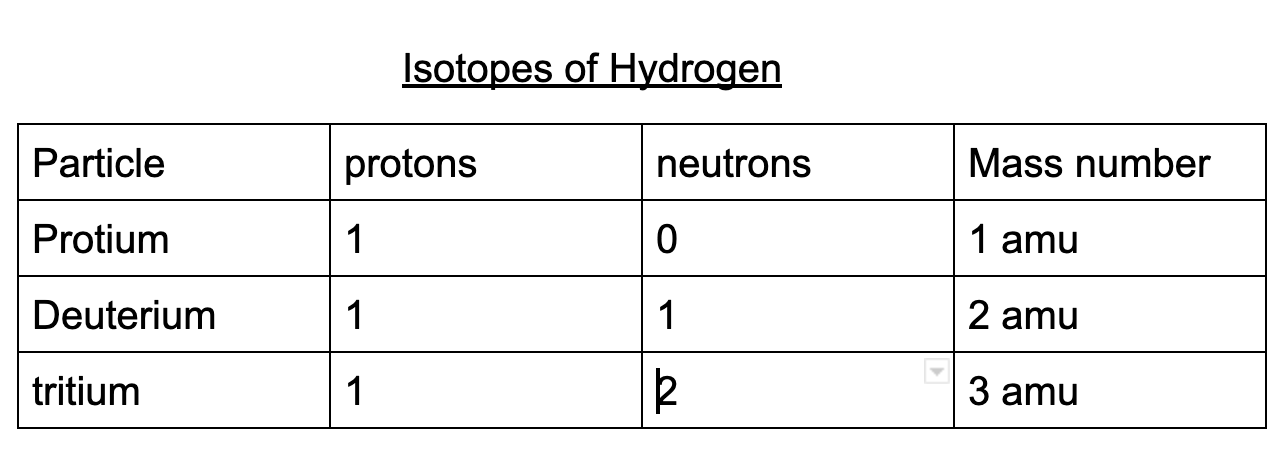
Isotopes: atoms of the same element that have different numbers of neutrons, hence have different Mass numbers.
Ex.
Atomic masses: are the average mass of all the Isotopes in a sample of the element