Chapter 4
The production of light is closely connect to atomic structure
What is Light?
Light is electromagnetic radiation
a form of energy
travels in waves
exists in increments called photons
- packet of light
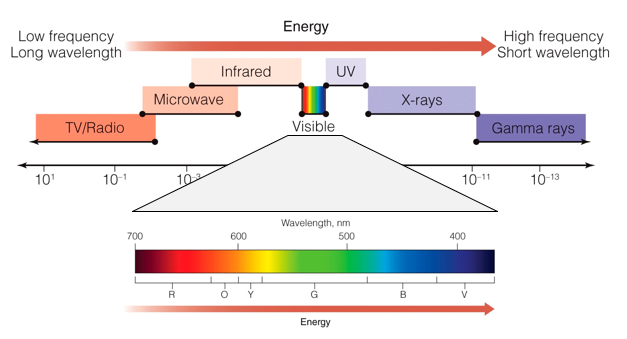
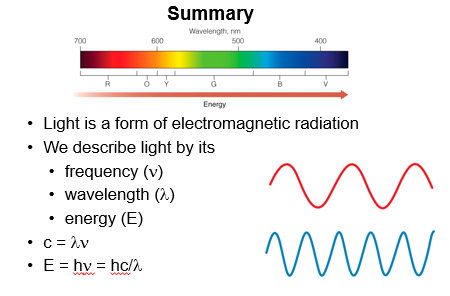
In the middle of the spectrum is a small range of radiation that our eyes can detect, and that we perceive as visible light. We can see that this spectrum is made up of the colors of the rainbow.
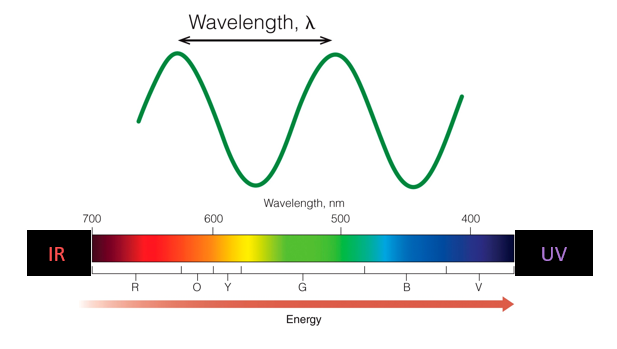
Wavelength is the distance from on one wave to the same point of the next wave and is used describe electromagnetic waves. It is usually measured meters or nanometers.
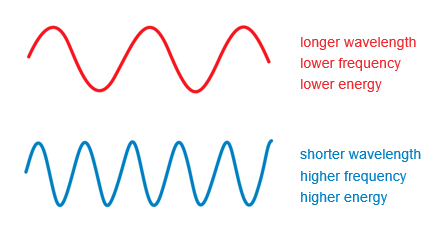
The color of light is related to its wavelength. On the low energy end of the visible spectrum, red light has a wavelength of about 700 nm. Wavelengths longer than this fall into the infrared region. On the high energy end of the visible spectrum, violet light has a wavelength of about 400 to 370 nm. Wavelengths shorts than this fall into the ultraviolet (or UV) region.
Describing Electromagnetic Waves

We often describe waves by their frequency. Frequency is the number of waves that pass through a point in one second. A frequency of one wave cycle per second is hertz.
Wavelength and frequency are inversely related to each other. When wavelength decreases, the frequency increases, and vice versa.
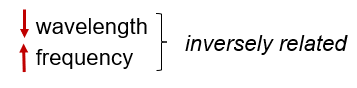
Mathematically, we describe this relationship as:

C = the speed of light
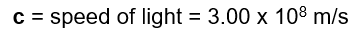
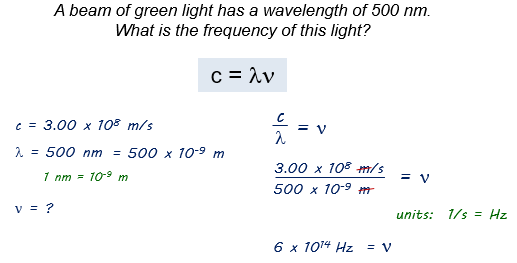
Here is an example:
The energy of light depends on its frequency and wavelength
We often describe light in terms of its energy. The energy of a photon of light depends on its frequency and wavelength.
Energy of a photon
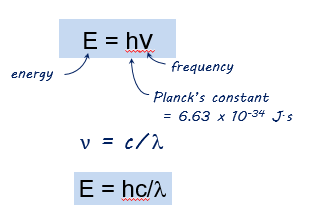
The h is Planck’s constant. This constant is named after Max Planck, a German physicist who was instrumental in developing the theories that relate light, energy, and electron structure. Because nu (V) is equal to c/lamda, we can also replace that with the V.
Here is an example:
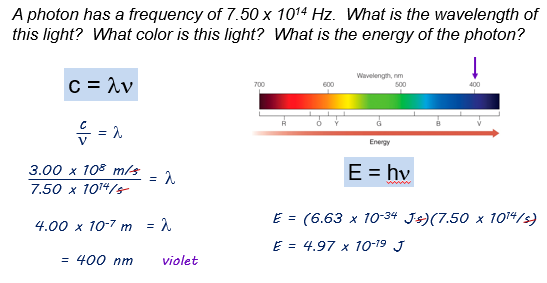
Summary:
Color, Line Spectra, and the Bohr Model
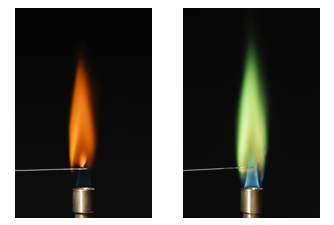
Flame test - Observe colors emitted by different metal ions
In this experiment, a wire is dipped in a solution containing metal ions. The ions of each element give off a characteristic color when heated.
Gas lamps also produce unique colors
These lamps produce light by passing an electric current through a tube filled with a gas such as helium, neon, argon, krypton, and xenon. Like the metals in a flame test, each gas in a lamp produces a characteristic color.
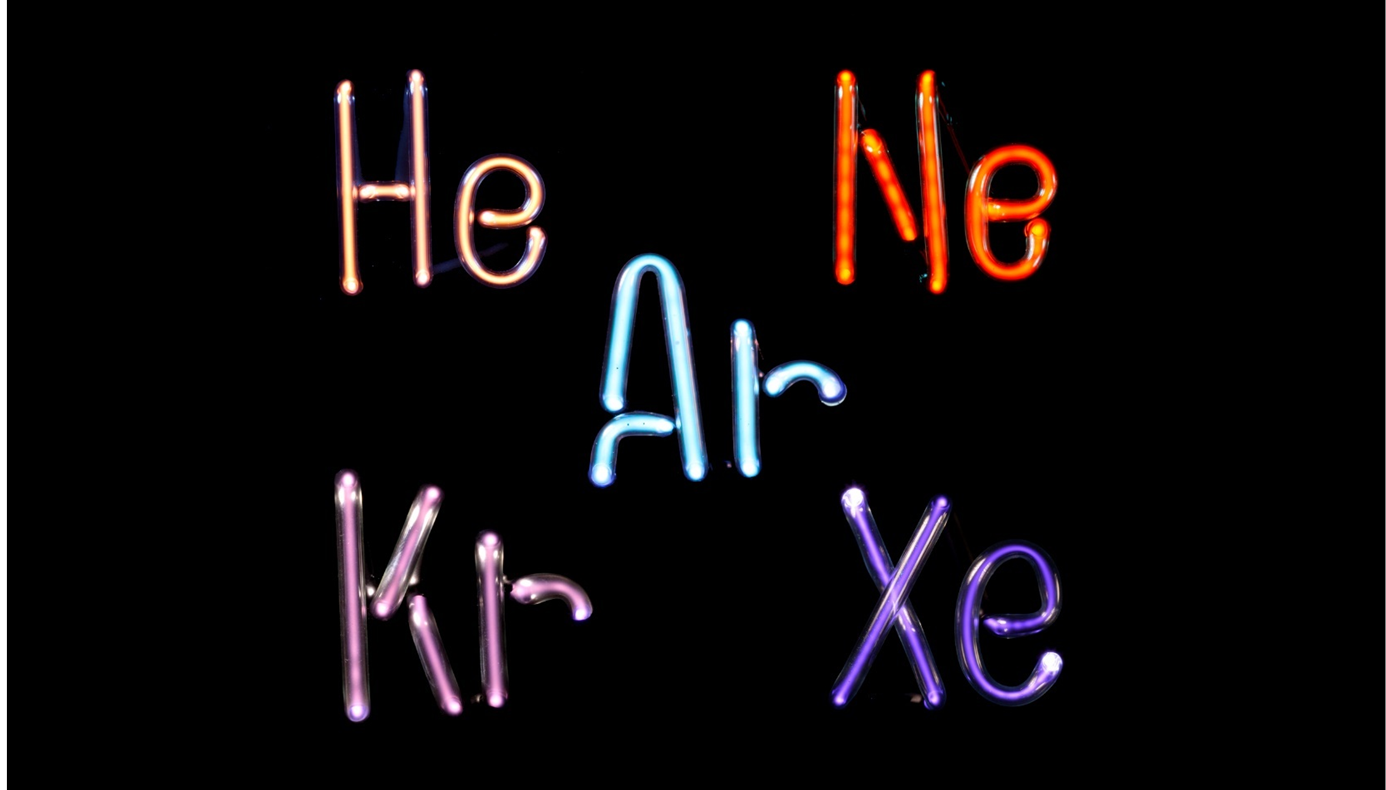
Line Spectra
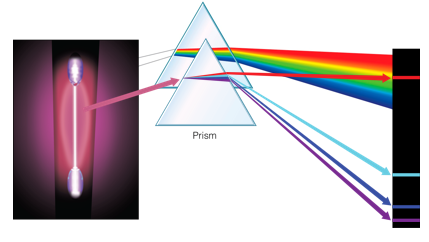
We can analyze the light from these lamps using a glass prism. When light passes through a prism, it separates into its constituent colors. Many white light sources produces all of the visible colors. If we pass this light through a prism, we see the complete rainbow of colors.
But if we do the same thing with light from a gas lamp, we see a fascinating result: Rather than producing a continuous spectrum of colors, gas lamps produce colors of only certain energies, called spectral lines.
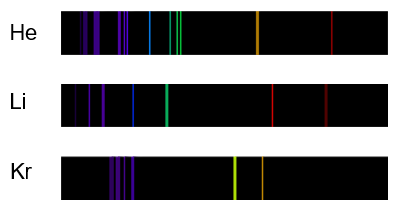
Each element produces a unique line spectrum
Scientists often use line spectra as “fingerprints” to identify elements. For example, when scientists analyze light from the sun, they find lines that correspond to the spectral lines of hydrogen and helium. From this, they know that the sun and other stars are composed largely of these two elements
Early 20th century:
- Dense nucleus surrounded by electrons
- Photoelectric effect: light causes atoms to eject electrons
The Bohr Model (1913)
Proposed by Niels Bohr
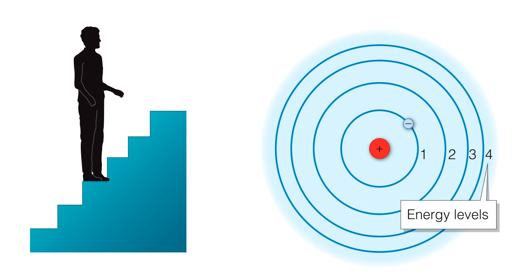
- Electrons orbit the nucleus
- Only certain orbit energies are “allowed”
- Electrons can jump between levels
- Light is absorbed or released when electrons jump
- When an electron absorbs light, it jumps to a higher energy level
- When it drops a lower energy level, it releases that energy as light
- If electrons jump to higher levels, the atom is in the excited state
Ground state: all electrons in lowest possible levels
Electrons can only occupy specified energy “steps”. Electrons can absorb energy to move to a higher level (step), or release energy to move to a lower level.
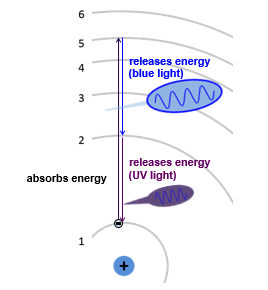
In its ground state, hydrogen’s one electron occupies the lowest possible level - energy level 1. However this electron can absorb energy (in the form of heat, light, or electrical energy). When this happens, the electron jumps from level 1 (the ground state) to a higher energy level (an excited state). Eventually, the electron relaxes for the excited state back down to lower energy states.
As it does so, it releases energy as electromagnetic radiation. For example, if the electron drops from level 5 to level 2, it releases energy in the form of a photon of blue light. As it dropped from level 2 to level 1, it releases ultraviolet light.
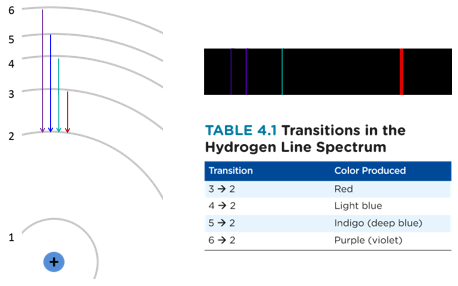
In a hydrogen atom, four transitions produce visible light, resulting in four spectral lines. Notice that the smallest transition produces the lowest energy light (red), while the biggest transition produces the highest energy light (purple). Other transition also take place, but they produce radiation that is either too high in energy (ultraviolet) or too low energy (infrared) for our eyes to detect.
Bohr’s ideas about light and electron energy levels were a critical advance, and provided a foundation for understanding the interplay of light and electrons that occurs all around us.
Other situations where light is produces
Nuclear fusion - The reason the sun gives off light
- Releases an incredible amount of energy
- Continually excited electrons (and other charged particles) which release this energy as electromagnetic radiation
The fire gives off light when a substance like wood burns, the carbon and hydrogen in the substance react with oxygen in the air.
- This reaction releases heat energy, as well as gaseous products such as carbon dioxide and water vapor.
- The electrons within these compounds are excited to higher levels. As these gaseous products exit the reaction, their electrons release this energy as visible light, resulting the appearance of a flame.
People don’t give off visible light, but your body produces heat, you release this energy in the form of infrared radiation.
- Specialized cameras can convert infrared energy into visible images, making it possible to “see” someone in the dark. This technique is called infrared imaging or thermal imaging.
The Bohr Model

The Quantum Model and Electron Orbitals
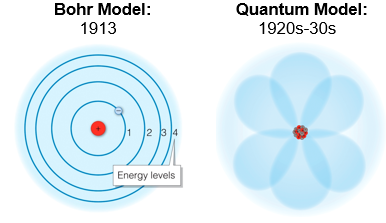
The Bohr Model proved useful for describing the chemical properties of the main - group elements elements, and the concept of electron energy levels was a key advance. However, Bohr’s model was unable to explain the many important properties, such as the behavior of the transition elements, or the complex line spectra of elements larger than hydrogen.
In the 1920s and 1930s, a series of discoveries led scientists to abandons much of the Bohr model in favor of a more nuanced description of electron behavior, called the quantum model, which describes electrons both as particles and as waves. These early discoveries marked the dawn of quantum mechanics, a field of study that deals with the unique and surprising behavior of subatomic particles.
Heisenberg’s Uncertainty Principle
In 1927, the German scientist Werner Heisenberg introduced a startling idea called the uncertainty principle. This principle deals with the mass, velocity, and location of a subatomic particles. One of the key ideas of this principle is that it is impossible to precisely know the exact velocity and location of a particle. Uncertainty becomes more significant as we deal with tiny, fast moving particles.
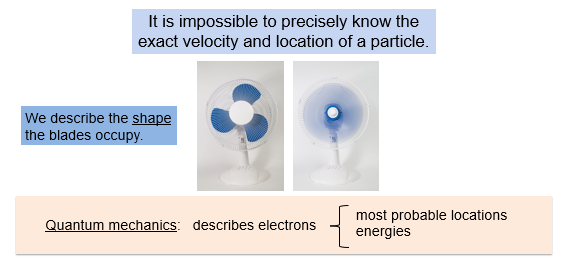
In quantum mechanics, we never talk about the exact position of an electron. According to Heisenberg, this is impossible for us to know. Instead, we talk about the most probable locations of the electrons or the energies that the electrons posses.
The wave nature of electrons
A second principle of quantum mechanics is equally surprising: When dealing with tiny particles (such as electrons), we can’t describe them simply as particles - many of their behaviors more closely resemble energy waves. The Bohr model postulated that only certain energies were allowed, but couldn’t explain why this was the case. If electrons are simply negatively charged particles, we can’t explain this phenomenon.

But what is electrons behave as waves? Many types of waves, oscillate at very specific energies. By treating electrons as energy waves, quantum mechanics is able to explain why electrons only exist at specific energy levels, and even predict the energy changes that produce line spectra.
The Quantum Model

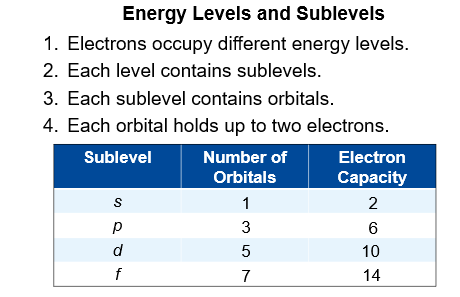
Energy Levels and Sublevels
Electrons occupy different energy levels.
- Level is identified by its principal quantum number, n (1,2,3..)
- Higher energy levels can hold more electrons
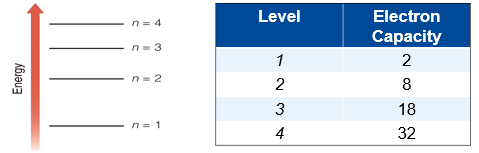
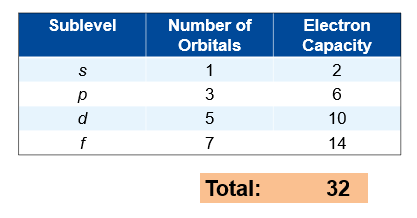
Each energy level contains one or more sublevels
Each sublevel contains one or more orbitals
- The term “orbitals” describes the region around the atom where the electron is most likely to be
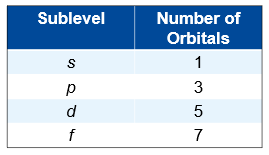
Each orbital holds up to two electrons
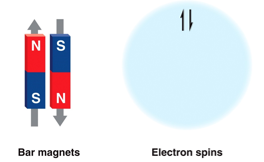
- Electrons have a magnetic field, called spin
- Electrons with opposite spins pair together
- An orbital is filled if it contains two paired electrons
Summary
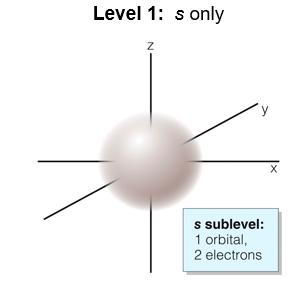
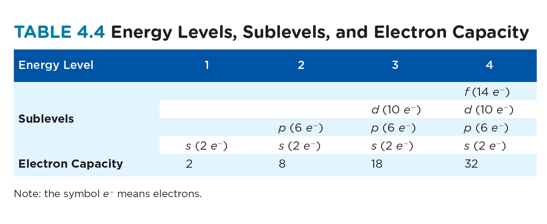
Level 1: s only
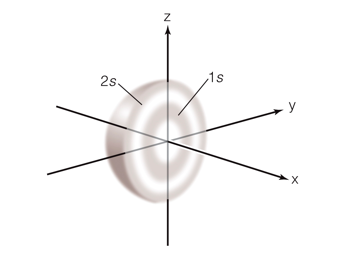
Level 2: s + p
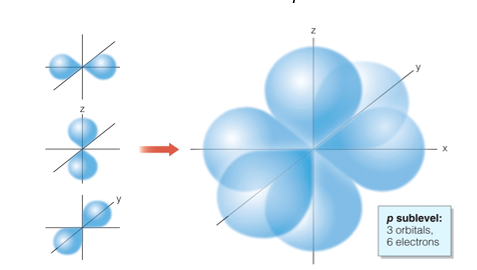
Level 3: s + p + d
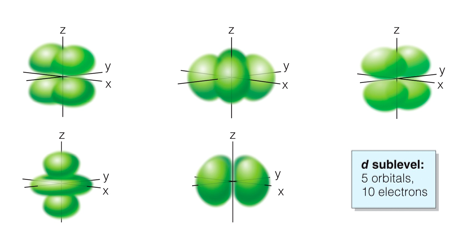

Level 4: s + p + d + f
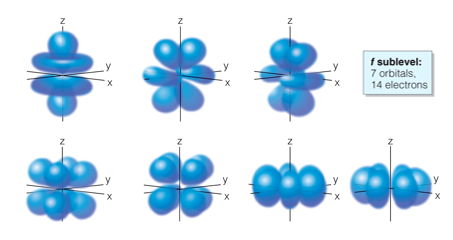
Summary
Describing Electron Configuration
 In order to understand how electrons fill the different energy levels and sublevels, let’s look at the electron configurations of a series of atoms. As we do so, remember that the atomic number, located on the periodic table, tells us the number of electrons present in any neutral atom
In order to understand how electrons fill the different energy levels and sublevels, let’s look at the electron configurations of a series of atoms. As we do so, remember that the atomic number, located on the periodic table, tells us the number of electrons present in any neutral atom
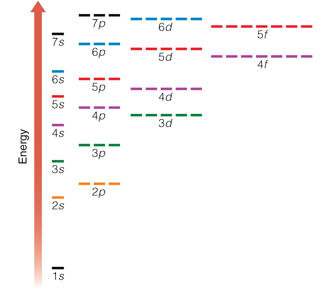
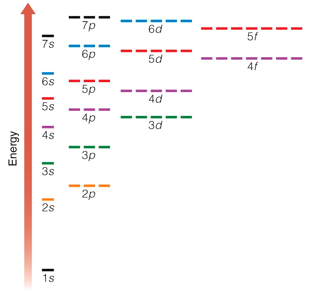
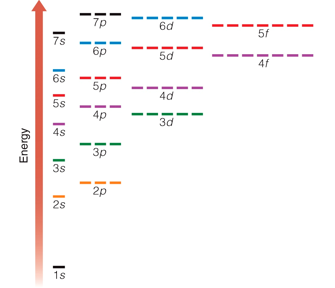
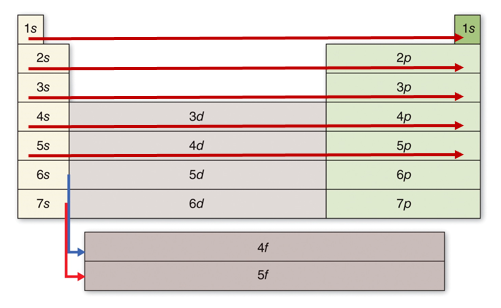
This diagram shows the relative energy differences between the different energy levels and sublevels. As a general rule, electrons occupy the lowest available energy levels - this is called the ground state.
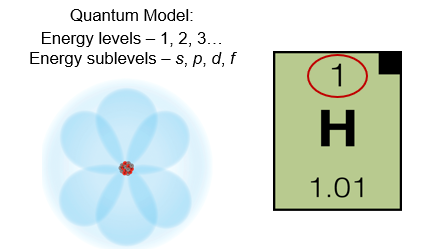
Hydrogen
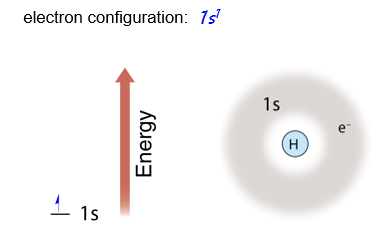
Let’s begin with hydrogen, which has only one electron. The single electron in hydrogen occupies the lowest energy level and sublevel possible. This electron occupies energy level 1, sublevel s. We represent this on an energy diagram by drawing a single - headed arrow in the 1s level.
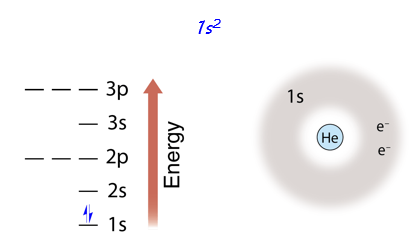
Helium
The next atom on the periodic table is helium, which has two electrons. Both electrons go in the lowest level and sublevel (1s), filling energy level 1. On the energy diagram, we represent two electrons in one orbital by drawing two arrows. One is drawn with the half - arrowhead up, and the other with the half - arrowhead down. This signifies that the spins (that is, the magnetic fields of the electrons) are oriented in opposite directions.
Hund’s Rule
Hund’s Rule - If empty orbitals of the same energy are available, electrons singly occupy orbitals rather than pairing together
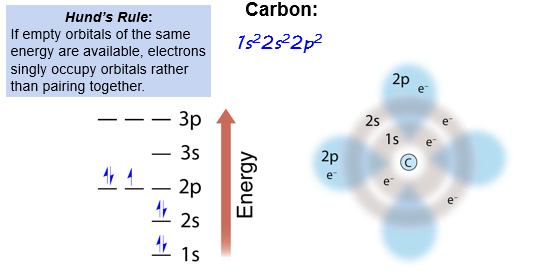
Continuing
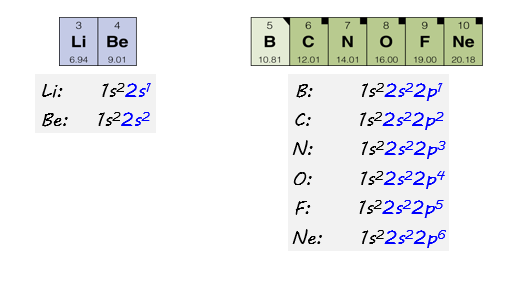
The electron configurations of the remaining elements in Row 2 of the periodic table are shown here. Notice that as we move across the periodic table in row 2, the second energy level fills with electrons. Neon, the last element in row 2, has a completely filled energy level
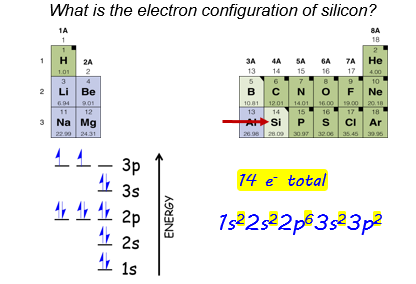
Let’s look at an example that extends this to row three. What is the electron configuration of silicon?
From the periodic table, we see that silicon is atomic number 14. This means there are 14 electrons in a neutral silicon atom.
We begin by placing two electrons in the lowest energy level (1s). Sublevel 2s can hold two more electrons. Sublevel 2p can hold six. The next lowest level is 3s, which can hold two electrons. The remaining two electrons go in next lowest level, which is 3p. Again, we follow Hund’s rule as we fill in the energy diagram. After you write the electron configuration, it’s a good idea to make sure the superscripts add up to the correct number of electrons: 2+2+6+2+2 = 14.
Notice that silicon is in row three, and its highest energy electrons occupy energy level three. The row of the periodic table corresponds to the highest - energy level electrons in the atom.
A diagram of electron energies like this one provides a guide for writing electron configurations. Remember that each orbital (signified by a horizontal line) can hold two electrons. Electrons fill the lowest energy levels possible.
Describing Electron Configuration (Part 2)
Valance Level: The highest occupied electron energy level
- Up to 8 electrons in valence level
Chemical changes involve the gain, loss, or sharing of electrons. Understanding the electron configuration enables us to explain and predict an element’s chemical properties
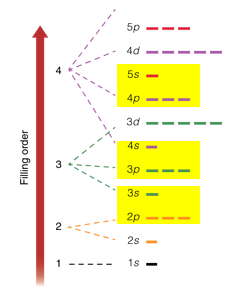
To see why atoms can only hold up to 8 atoms in their valence, consider this simplified representation of the electron energy levels. Notice that the level above 2p is 3s. The level above 3p is 4s, and the level above 4p is 5s. If an atom has its s and p sublevels filled, the next electron goes into a higher energy level.
For example, argon has 18 electrons. Therefore, we write its electron configuration as 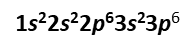
An argon atom has 8 electrons in its valence level (energy level three). The next atom on the periodic table, potassium, has an electron configuration of 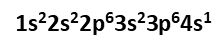 Potassium has one electron in its valence level (energy level four).
Potassium has one electron in its valence level (energy level four).
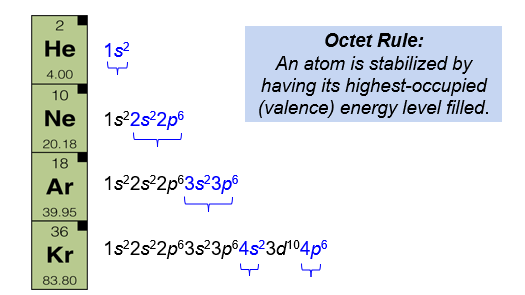
 Noble Gases have Filled Valences
Noble Gases have Filled Valences
Octet Rule: An atom is stabilized by having its highest - occupied (valence) energy level filled.
The noble gases, located on the far - right column of the periodic table, have completely filled valence levels. These elements are stable and unreactive - they generally do not combine with other atoms to form compounds. We describe this stability using the octet rule. If an atom has eight electrons (an octet) in its valence shell, the s and p sublevels are completely filled.
Electron Configurations for Larger Atoms
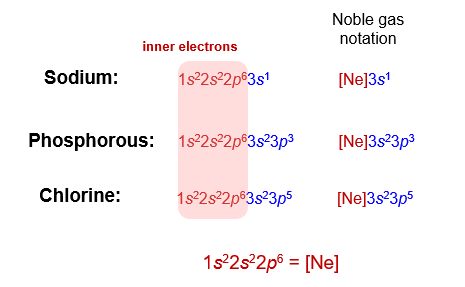
Notice that in each of these configurations, the first two levels (sometimes called the inner electrons) do not change. If we add electrons, the change only affects the valence level. Since the inner electrons do not change, we often represent them in a simpler form, called the noble gas notation.
When using this notation, we always use the noble gases, which have complete octets in their inner levels.

In addition to convenience, there is another advantage to using the noble gas notation. This notation allows us to focus on outer electrons (those beyond the largest filled noble gas configuration). The outer electrons include the valence level and partially filled d and f sublevels. The formation of chemical bonds involve changes in the outer electrons. The inner electrons are not involved with bonding.
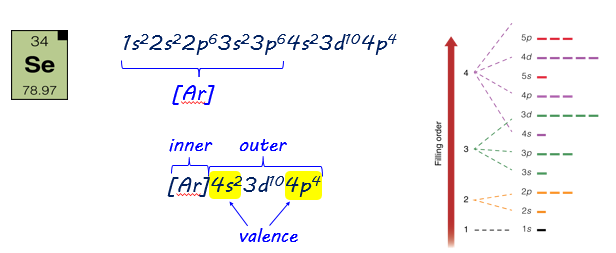
The valence electrons are only those in the highest energy level - that is, energy level 4, this example. Selenium has 6 valence electrons.
Electron Configurations for Ions
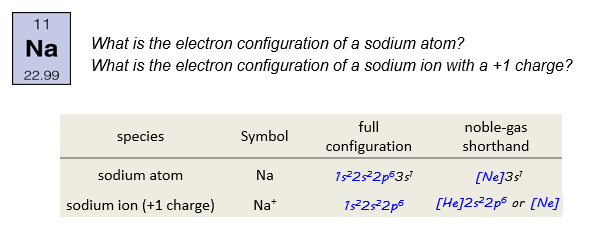
Recall that ions are particles with an overall positive or negative charge. Ions are a vital part of chemistry, and it is important to understand their electron configurations.
The sodium ion has a charge of +1, so it must have one electron fewer than neutral sodium. The electron that is removed is from the valence energy level (3s). This leaves us with an electron configuration of 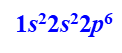
The electron configuration of the sodium ion is identical to that of neon, which has energy level two completely filled. We could write this in the noble gas shorthand as [Ne].
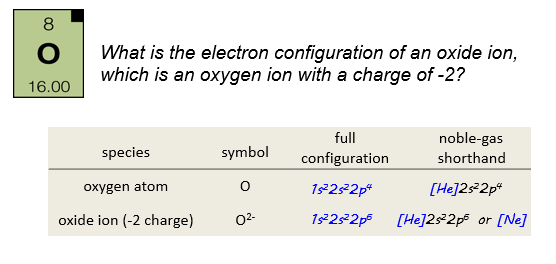
The oxide ion has a charge of -2, so it must have two more electrons than the neutral oxygen. The electrons that are gained by the atom fill the lowest available energy shell. We could write this in noble gas shorthand as, simply, [Ne].
Many ions form noble gas configurations

Isoelectronic - they have the same electron configurations
The gain, loss, and sharing of electrons is central to understand chemistry.
Electron Configuration and the Periodic Table
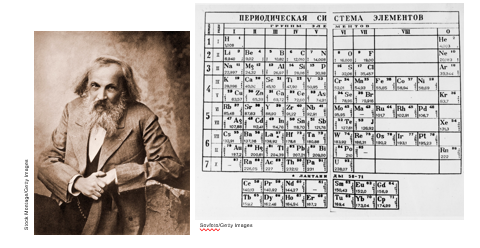 In the late 1800s, Dmitri Mendeleev organized the periodic table, based on the chemical and physical properties of the elements. Many of these properties depend on an element’s electron configuration.
In the late 1800s, Dmitri Mendeleev organized the periodic table, based on the chemical and physical properties of the elements. Many of these properties depend on an element’s electron configuration.
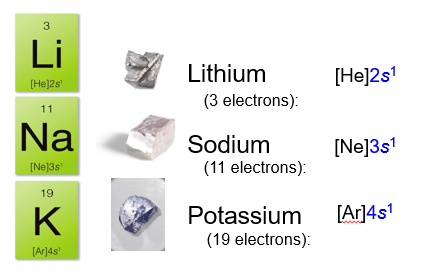
All of these react quickly, even violently with water, and form ions with a +1 charge.
Notice that these atoms all have an s1 configuration in their valence level. These elements exhibit similar behaviors because their electron configurations are similar.
On the periodic table, these elements are located in a single column, on the far left hand side of the table.

Fluorine, chlorine, and bromine all form ions with a charge of -1.
In their valence level, they all have a configuration of 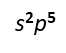
Just as in the previous example, they are located in a single column, but this time on the right hand side of the periodic table.
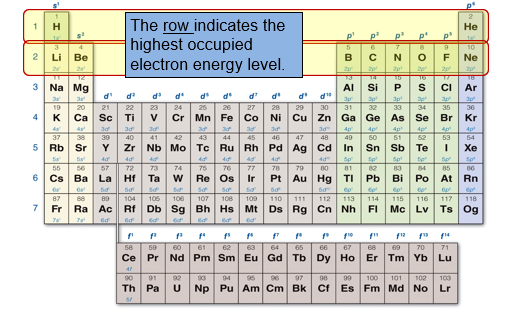
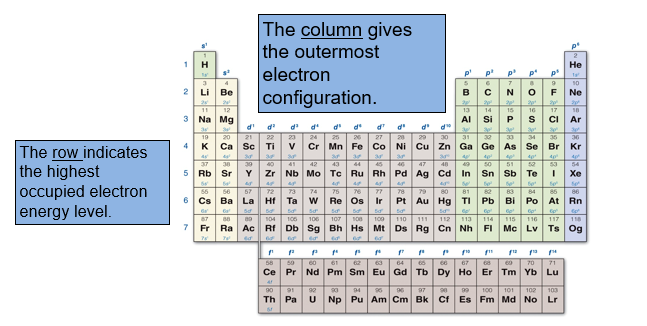
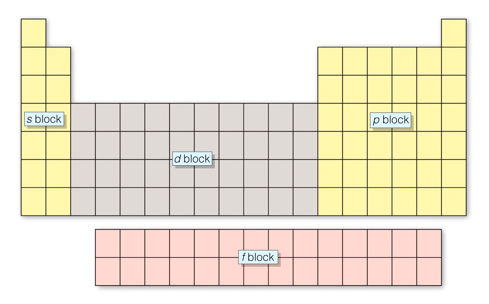
The periodic table is organized based on element masses and properties, but it is also organized based on electronic structure. For any atom, we can determine its highest occupied electron energy level from the row it occupies on the periodic table.
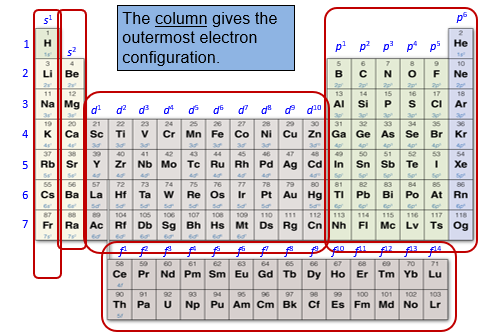
The columns on the periodic table correspond to the outermost or sublevel electron configuration.
Every atom in the first column has an electron configuration of s1, every atom in the second column has an electron configuration of s2.
The right - hand main group block contain elements with highest energy electrons in a p sublevel. The columns correspond to elements having a p1 - p6 electron configurations.
The transition elements have outer electron configurations corresponding to d1 to d10.
The inner transition elements have out electron configurations corresponding to f1 to f14.
Summary