CHAPTER 12: SOLIDS AND MODERN MATERIALS
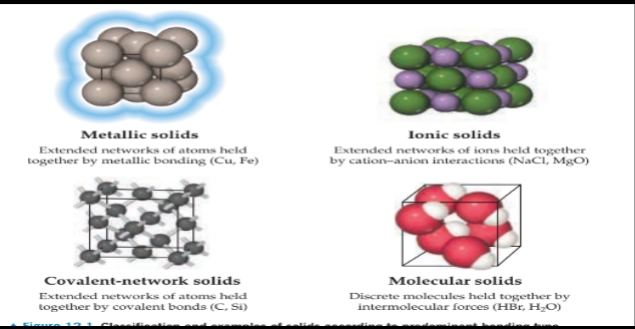
Classification of Solids
Metallic solids
- Are held together by a delocalized “sea” of collectively shared valence electrons. This form of bonding
- Allows metals to conduct electricity. It is also responsible for the fact that most metals are relatively strong without being brittle.
Ionic solids
- Are held together by the mutual electrostatic attraction between cations and anions. Differences between ionic and metallic bonding make the electrical and mechanical properties of ionic solids very different from those of metals: Ionic solids do not conduct electricity well and are brittle.
Covalent-network solids
- Are held together by an extended network of covalent bonds. This type of bonding can result in materials that are extremely hard, like diamond, and it is also responsible for the unique properties of semiconductors.
Molecular solids
- Are held together by the intermolecular forces.
Polymers
- Contain long chains of atoms (usually carbon), where the atoms within a given chain are connected by covalent bonds and adjacent chains are held to one another largely by weaker intermolecular forces.
Nanomaterials
Are solids in which the dimensions of individual crystals have been reduced to the order of 1–100 nm.
Structures of Solids
Crystalline solids
- Solids in which atoms are arranged in an orderly repeating pattern.
- These solids usually have flat surfaces, or faces, that make definite angles with one another.
- Examples of crystalline solids include sodium chloride, quartz, and diamond.
Amorphous solids
- (from the Greek words for “without form”) lack the order found in crystalline solids.
- Amorphous solids do not have the well-defined faces and shapes of a crystal.
- Examples of crystalline and amorphous solids. The atoms in crystalline solids repeat in an orderly, periodic fashion that leads to well-defined faces at the macroscopic level. This order is lacking in amorphous solids like obsidian (volcanic glass).
Unit cell
- Is a relatively small repeating unit.
Crystal lattice
- The geometrical pattern of points on which the unit cells are arranged.
Lattice vectors
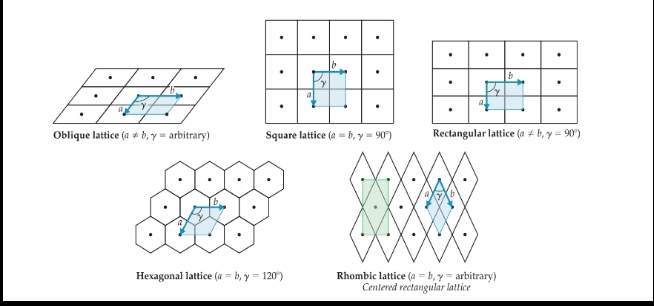
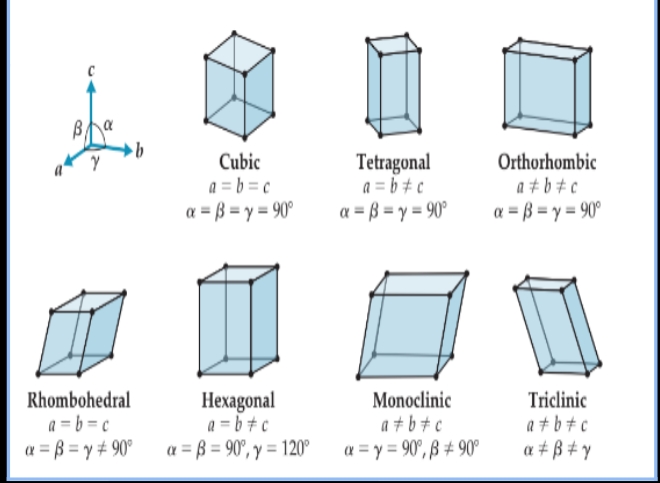
- The positions of the lattice points.
The seven three-dimensional primitive lattices.
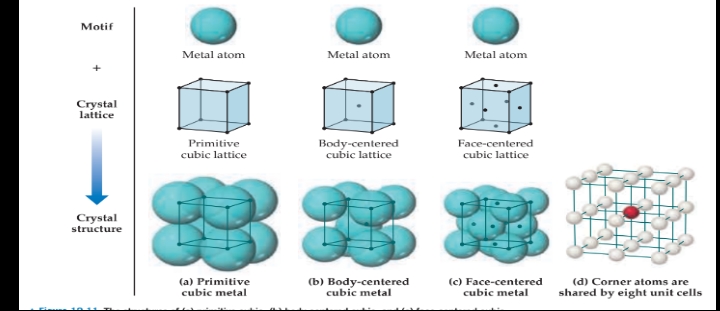
The three types of cubic lattices:
Primitive cubic lattice
Lattice points only at corners.
\n Body-centered cubic lattice
Lattice points at corners plus one lattice point in the center of unit cell.
Face-centered cubic lattice
- Lattice points at corners plus one lattice point at the center of each face.
Metallic Solids
Metallic solids
- [ ] Also simply called metals, consist entirely of metal atoms. The bonding in metals is too strong to be due to dispersion forces, and yet there are not enough valence electrons to form covalent bonds between atoms.
Metallic bonding
- Happens because the valence electrons are delocalized throughout the entire solid.
The Structures of Metallic Solids
\n Close Packing
Hexagonal close packing (hcp)
- Spheres sit in depressions marked with yellow dots.
- Spheres sit in depressions that lie directly over spheres of first layer, ABAB stacking.
Cubic close packing (ccp)
- Spheres sit in depressions marked with yellow dots.
- Spheres sit in depressions marked with red dots; centers of third-layer spheres offset from centers of spheres in first two layers, ABCABC stacking. \n
Alloys
- Is a material that contains more than one element and has the characteristic properties of a metal.
- Alloys can be divided into four categories: substitutional alloys, interstitial alloys, heterogeneous alloys, and intermetallic compounds.
Substitutional alloy
- When atoms of the solute in a solid solution occupy positions normally occupied by a solvent atom.
Interstitial alloy
When the solute atoms occupy interstitial positions in the “holes” between solvent atoms.
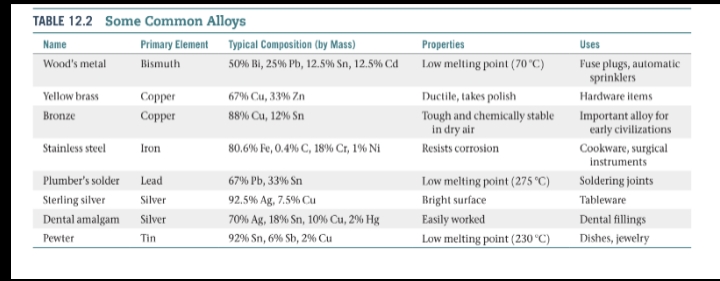
Heterogeneous alloy
- The components are not dispersed uniformly.
Intermetallic compounds
- Are compounds rather than mixtures.
Metallic Bonding
Electron-Sea Model
- [ ] A simple model that accounts for some of the most important characteristics of metals which pictures the metal as an array of metal cations in a “sea” of valence electrons.
Molecular Orbital Model
Rules of molecular orbital theory:
- Atomic orbitals combine to make molecular orbitals that can extend over the entire molecule.
- A molecular orbital can contain zero, one, or two electrons.
- The number of molecular orbitals in a molecule equals the number of atomic orbitals that combine to form molecular orbitals.
- Adding electrons to a bonding molecular orbital strengthens bonding, while adding electrons to antibonding molecular orbitals weakens bonding.
Electronic Band Structure
- [ ] If the chain becomes very long, there are so many molecular orbitals that the energy separation between them becomes vanishingly small. As the chain length goes to infinity, the allowed energy states become a continuous band.
Band structure
- The electronic structure of a bulk solid.
Ionic Solids
Ionic solids
- are held together by the electrostatic attraction between cations and anions: ionic bonds.
Structures of Ionic Solids
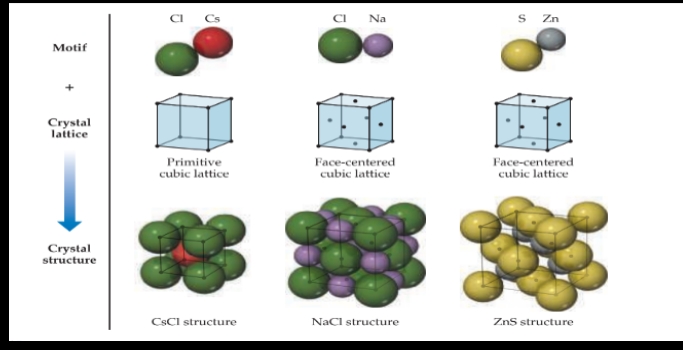
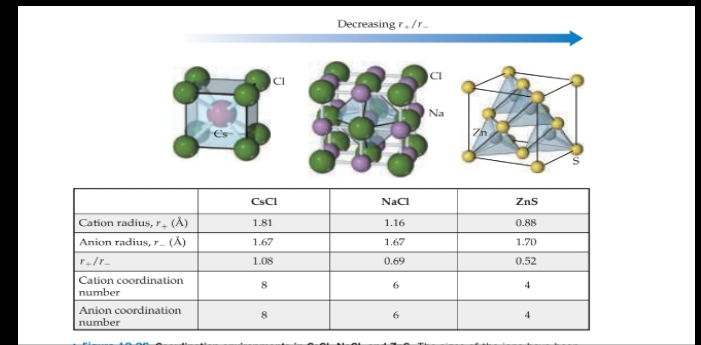
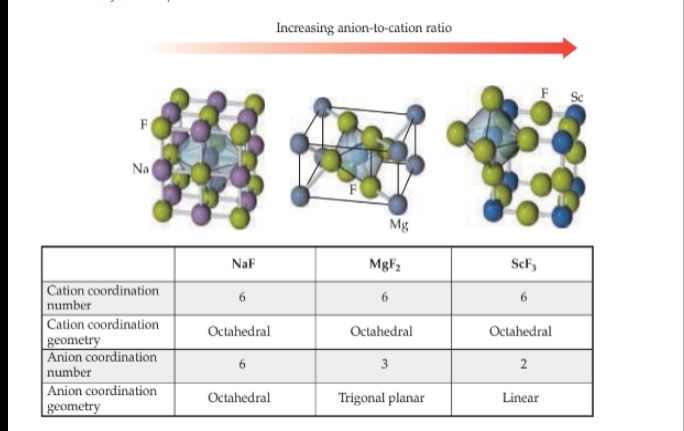
Molecular Solids
Molecular solids
- Consist of atoms or neutral molecules held together by dipole–dipole forces, dispersion forces, and/or hydrogen bonds.
- The properties of molecular solids depend in large part on the strengths of the forces between molecules.
- Molecular shape is also important because it dictates how efficiently molecules pack together in three dimensions. \n
Covalent-Network Solids
Covalent-network solids
- [ ] Consist of atoms held together in large networks by covalent bonds. Because covalent bonds are much stronger than intermolecular forces, these solids are much harder and have higher melting points than molecular solids.
Semiconductors
- Metals conduct electricity extremely well. Many solids, however, conduct electricity somewhat, but nowhere near as well as metals.
- Two examples of semiconductors are silicon and germanium.
Valence band
- The band that forms from bonding molecular orbitals.
Conduction band
- The band that forms the antibonding orbitals.
Insulator
- Does not conduct electricity.
Semiconductor doping
Doping
- The process of adding controlled amounts of impurity atoms to a material.
- Doping provides an opportunity for controlling the electrical conductivity through precise control of the type and concentration of dopants. \n
Polymers
- [ ] In 1827 Jöns Jakob Berzelius coined the word polymer (from the Greek polys, “many,” and meros, “parts”) to denote molecular substances of high molecular weight formed by the polymerization (joining together) of monomers, molecules with low molecular weight.
Plastics
- Are polymeric solids that can be formed into various shapes, usually by the application of heat and pressure.
Thermoplastics
- Can be reshaped. For example, plastic milk containers are made from thermoplastic polymer polyethylene.
Thermosetting plastic
- (also called a thermoset) is shaped through irreversible chemical processes and, therefore, cannot be reshaped readily.
Elastomer
- Which is a material that exhibits rubbery or elastic behavior.
Making Polymers
Addition polymerization
- This type of polymerization, in which monomers are coupled through their multiple bonds.
Condensation polymerization
- A second general reaction used to synthesize commercially important polymers.
Copolymers
- Polymers formed from two different monomers.
Structure and Physical Properties of Polymers
Cross-linking
- Forming bonds between chains.
- An important example of cross-linking is the vulcanization of natural rubber, a process discovered by Charles Goodyear in 1839.