Note
0.0(0)
Explore Top Notes Note
Note Studied by 20 people
Studied by 20 people Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 110 people
Studied by 110 people Note
Note Studied by 18 people
Studied by 18 people Note
Note Studied by 20 people
Studied by 20 people Note
Note Studied by 12 people
Studied by 12 people
Characteristics of Life Vocab/Notes
5.0(1)
Scientists of EM Theory and Applications of Electromagnetic
5.0(2)
APUSH: REVIEW OF UNIT 1
5.0(1)
AP Psych Notes
5.0(1)
Intro to Sociology and Sociological Imagination
5.0(1)
Chapter 10: Electrons and Electronic
5.0(1)
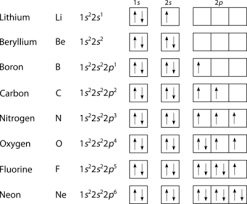
Electron shells and atomic orbitals
Electron shells:
- Formula for the number of electrons each shell can hold 2n^2
- Electrons orbit the nucleus in energy levels or electron shells.
- The energy level is determined by the principal quantum number (n).
- The larger the value for n the further away from the nucleus the shell is and the higher the energy.
| n (quantum number) | shell | number of electrons |
|---|---|---|
| 1 | 1st | 2 |
| 2 | 2nd | 8 |
| 3 | 3rd | 18 |
| 4 | 4th | 32 |
Atomic orbitals:
- Only two electrons can ever be in a single orbital
- The two electrons must have opposite spins
The s-orbitals:
- Spherical in shape
- All shells contain an s-orbital
- S-orbitals can hold 2 electrons
The p-orbitals:
- 3D dumbbell shape
- All shells from n=2 upwards contain 3 p-orbitals
- There are three p orbitals px, py and pz
D and f-orbitals:
- 5 d-orbitals (from n=3 upwards)
- 7 f-orbitals (from n=4 upwards)
| Shell (quantum no.) | s (no. of orbitals) | p | d | f | Max electrons |
|---|---|---|---|---|---|
| 1 | 1 | N/A | N/A | N/A | 2 |
| 2 | 1 | 3 | N/A | N/A | 8 |
| 3 | 1 | 3 | 5 | N/A | 18 |
| 4 | 1 | 3 | 5 | 7 | 32 |
Filling orbitals:
Lowest energy orbitals are filled first
Sub-shells are filled singly first
When all equal energy sub-shells contain one electron they pair up with another electron with an opposite spin
Note
0.0(0)
Explore Top Notes Note
Note Studied by 20 people
Studied by 20 people Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 110 people
Studied by 110 people Note
Note Studied by 18 people
Studied by 18 people Note
Note Studied by 20 people
Studied by 20 people Note
Note Studied by 12 people
Studied by 12 people
Characteristics of Life Vocab/Notes
5.0(1)
Scientists of EM Theory and Applications of Electromagnetic
5.0(2)
APUSH: REVIEW OF UNIT 1
5.0(1)
AP Psych Notes
5.0(1)
Intro to Sociology and Sociological Imagination
5.0(1)
Chapter 10: Electrons and Electronic
5.0(1)