Quantity of Heat and Heat Transfer
Heat is a form of energy
Add heat energy → Temperature rise
Remove heat energy → Temperature falls
If a substance is changing state, there might not be any change in temperature.
Heat Capacity
The heat capacity (C) of an object is the amount of heat energy needed to change the temperature by 1K (1 oC)
SI Unit of Heat Capacity: joule per kelvin (J K-1)
Heat Capacity Formula
• Q joules of heat energy are added to an object.
• Its heat capacity is C joules per kelvin.
• Its temperature rises by ΔΘ degrees celsius.
heat energy need to change temperature = heat capacity x change in temperature
Q = C ΔΘ
Formula for Heat Energy (Heat Capacity)
Specific Heat Capacity
The specific heat capacity (c) of a substance is the heat energy needed to change the temperature of one kilogram of that substance by one kelvin.
SI Unit of Specific Heat Capacity: joule per kilogram per kelvin (J kg-1 K-1)
Specific Heat Capacity Formula
Provided a substance does not change state when heat energy is added to or taken from it we have:
heat energy added = mass x specific heat capacity x rise in temperature
heat energy lost = mass x specific heat capacity x fall in temperature
Q = m c ΔΘ
Formula for Heat Energy (Specific Heat Capacity)
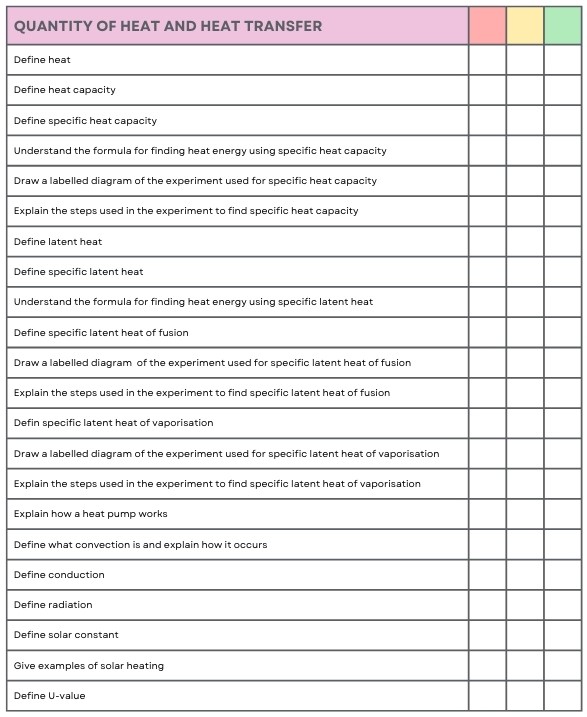
Latent Heat
Latent Heat (L)
The heat energy needed to change the state of a substance without a change in temperature.
SI Unit - joule (J)
Specific Latent Heat (l)
The heat energy needed to change the state of 1 kg of a substance without a change in temperature.
SI Unit - joule per kilogram (J kg-1)
Q = m l
heat energy needed to change state = mass x specific latent heat
Specific Latent Heat of Fusion
The heat energy needed to change 1 kg of a substance from a solid to a liquid without a change in temperature.
Specific Latent Heat of Vaporisation
The heat energy needed to change 1 kg of a substance from a liquid to a gas without a change in temperature.
e.g as perspiration evaporates, it takes its latent heat from the person and their body
temperature falls.
Application of Specific Latent Heat
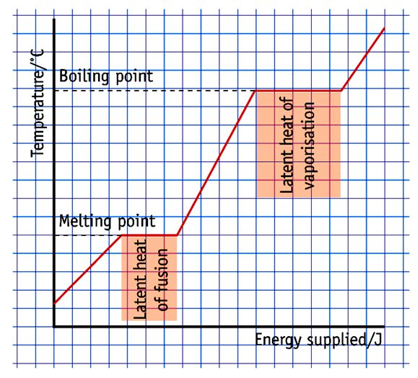
Heat Pump e.g. Fridge
In a heat pump, there is a low pressure liquid e.g. freon.
As it moves around, it absorbs heat from the inside.
This heat turns the low pressure liquid into a low pressure vapour.
Low pressure vapour goes through a compressor. This turns it into a high pressure vapour.
The high pressure vapour condenses into a high pressure liquid and gives off heat at the back of the fridge.
This high pressure liquid goes through an expansion valve and returns to a low pressure liquid as it was previously.
The cycle continues.
Heat Transfer
Convection
The movement of heat through a fluid by means of circulating currents caused by the heat.
Occurs when the lower part of the liquid is heated, it expands and becomes less dense
Less dense objects float on denser objects
Therefore heated liquid rises above cooler liquid
Conduction
The transfer of heat through a substance by the passing on of vibration from molecule to molecule. There is no overall motion of the substance.
Radiation
the transfer of heat energy from one place to another in the form of electromagnetic waves.
Solar Constant (Solar Irradiance)
The average amount of the sun’s energy falling per second perpendicularly on 1 metre squared of the Earth’s atmosphere.
It is roughly 1.35 kW m-2
Solar Heating
Using the suns energy to heat something
e.g. Solar Panels
Photocells convert suns energy into electrical energy
U-Value
The amount of heat energy conducted per second through 1m2 of that structure when the difference of 1oC is maintained between its ends.
SI Unit: W m-2 K-1
High U-Value = Poor Insulation
Low U-Value = Good Insulation
Learning Checklist