Recording-2025-03-08T12:06:22.157Z
Micromolecules and Macromolecules
Micromolecules are essential molecular components that make up most substances.
Example: Proteins are large molecules composed of hundreds to thousands of smaller units.
Classes of Macromolecules
Macromolecules are large molecules divided into four classes:
Carbohydrates
Lipids
Proteins
Nucleic acids
Macromolecules are referred to as polymers, formed by linking smaller units called monomers.
Example: A string of beads represents a polymer, with each bead symbolizing a monomer.
Monomers and Polymers
Three classes (carbohydrates, proteins, nucleic acids) are true polymers.
Lipids are not true polymers.
Polymers are made by dehydration synthesis, a reaction that joins monomers together while releasing water (dehydration reaction).
Hydrolysis reaction is the breakdown of polymers into monomers using water.
Digestive Process
Polymers are too large to be absorbed by the intestines; they must be broken down into smaller monomers.
Enzymes play a crucial role in this process:
Break down polysaccharides into monosaccharides.
Break down proteins into amino acids.
After digestion, monomers are absorbed into the bloodstream and can be reassembled into larger molecules as needed.
Carbohydrates
Carbohydrates include sugars and their polymers.
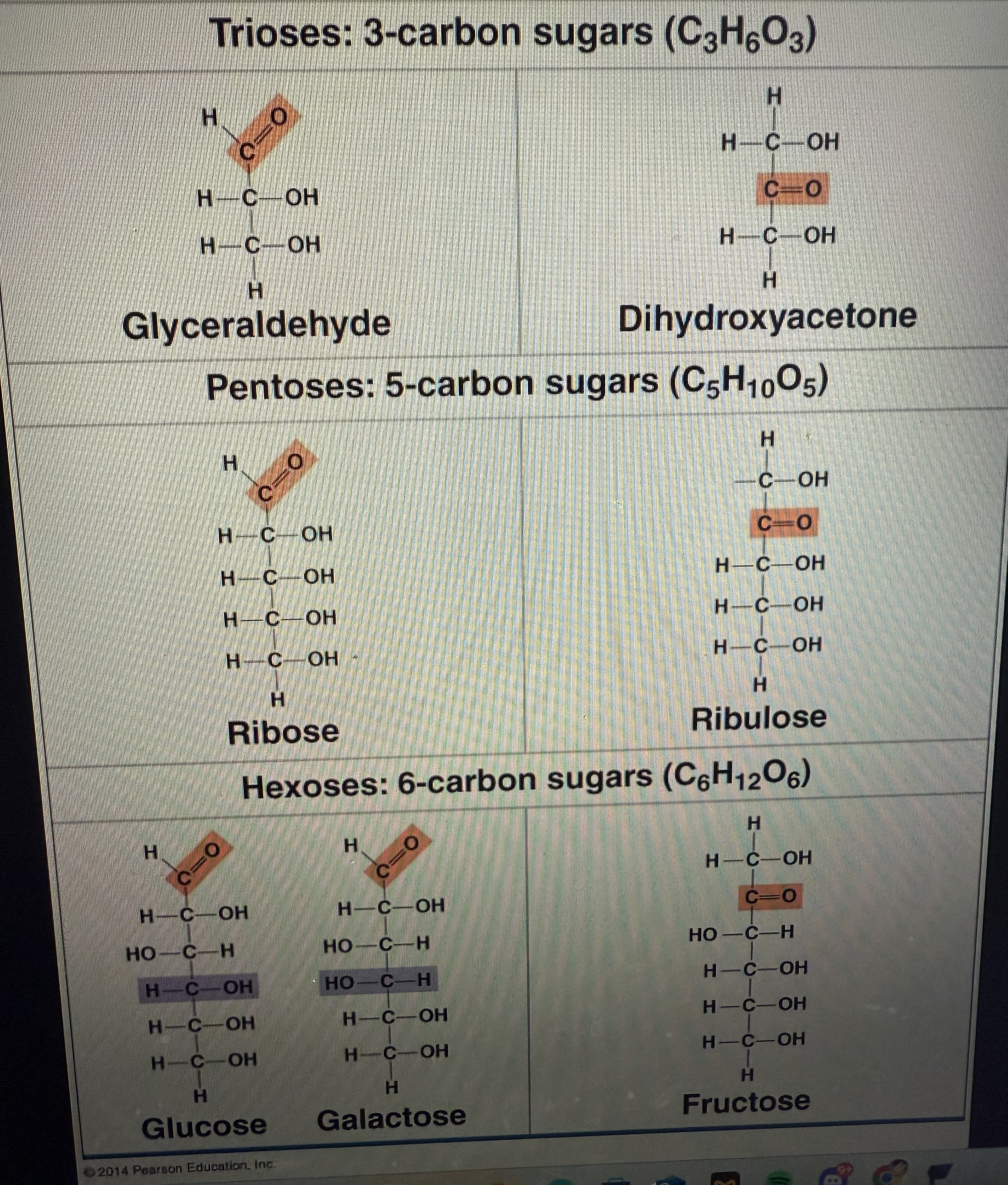
The simplest form is monosaccharides (e.g., glucose - C6H12O6).
Glucose is the most commmon monosaccharide and is the centrral importance in the chemistry of life
Glucose is an aldose
Monosaccharides are classified by:
Location of the carbonyl group (aldehyde or ketone).
Number of carbons (trioses, pentoses, hexoses).
Disaccharides are formed by joining two monosaccharides through covalent bonds (e.g., sucrose, lactose, maltose).
Polysaccharides
Polysaccharides are long chains of monosaccharides, used for energy storage (e.g., starch, glycogen) and structural support (e.g., cellulose).
Starch has two forms:
Amylose: A linear chain of glucose.
Amylopectin: A branched chain of glucose units.
Cellulose: A major structural component in plant cell walls, composed of beta-glucose which cannot be digested by humans but is digested by microbes in some animals.
Lipids
Lipids are a diverse group of biological molecules that do not mix with water, characterized by:
Hydrophobic nature due to non-polar covalent bonds.
Main types of lipids include:
Fats (glycerol and fatty acids), Phospholipids, and Steroids.
Fats are formed by linking three fatty acids to glycerol via dehydration reactions; these are called triacylglycerols.
Fatty acids can be:
Saturated (no double bonds) - typically solid at room temperature (e.g., butter).
Unsaturated (one or more double bonds) - typically liquid at room temperature (e.g., oils).
Phospholipids
Phospholipids have hydrophilic heads and hydrophobic tails, forming lipid bilayers in water, crucial for cell membranes.
The cell membrane structure is vital for the existence of life.
This lipid forms he main structural component of cell membranes
Steroids
Steroids are characterized by a carbon skeleton consisting of four fused rings (e.g., cholesterol).
Cholesterol is a precursor for important steroids such as testosterone.
SUGARS
carbonyl group (cooh) that forms ring structure with a hydroxyl group that forms ring structure when the dry molecule is placed in water
 Knowt
Knowt