AP Chem Unit 5
5.1 Reaction Rates / 5.5 Collision Theory
Chemical Kinetics: the area of chemistry concerned with the speed or rates, at which chemical reactions occur
Collision Theory: All reactions take place because of successful collisions between atoms or molecules. Successful collisions require sufficient energy and correct orientation
Collisions Theory Notes
single replacement reaction
high temp = more collisions
reactants move faster
low temp = less collisions
reactants move slower
reactant concentration increase = reactant percentage increase
some nature of reactants cause faster reaction
less collisions if less surface area of reactant
sugar cube v spoon of sugar
adding inert gas does not impact rage
gas not part of reaction mechanisms
Factors That Affect Rates
nature of reactants
surface area of a solid
concentration of reactants
temperature at which the reaction takes place
presence of catalyst
on both sides of equation
5.2 Reaction Rates
rate = k[A]m[B]n
k = rate constant
specific to experiment
get only from data
[A] and [B] = concentration of …
m and n = orders
bigger order = bigger impact
get from data only
5.4 Elementary Reactions
an elementary reaction is a process in a chemical reaction that occurs in a single event of step
an overall chemical ratio consists of one or more elementary steps
cross out things that are products and reactants across each step to get overall equation
slowest step determines rate
Rate Laws
rate law for an overall reaction must be determined using experimental data
in absence of data, rate law for an elementary reaction can be inferred from stoichiometry of the molecules participating in the collision
elementary reactions involving simultaneous collisions of three or more particles are rare so they are ignored
number of particles that collide during an elementary reaction is known as reaction molecularity
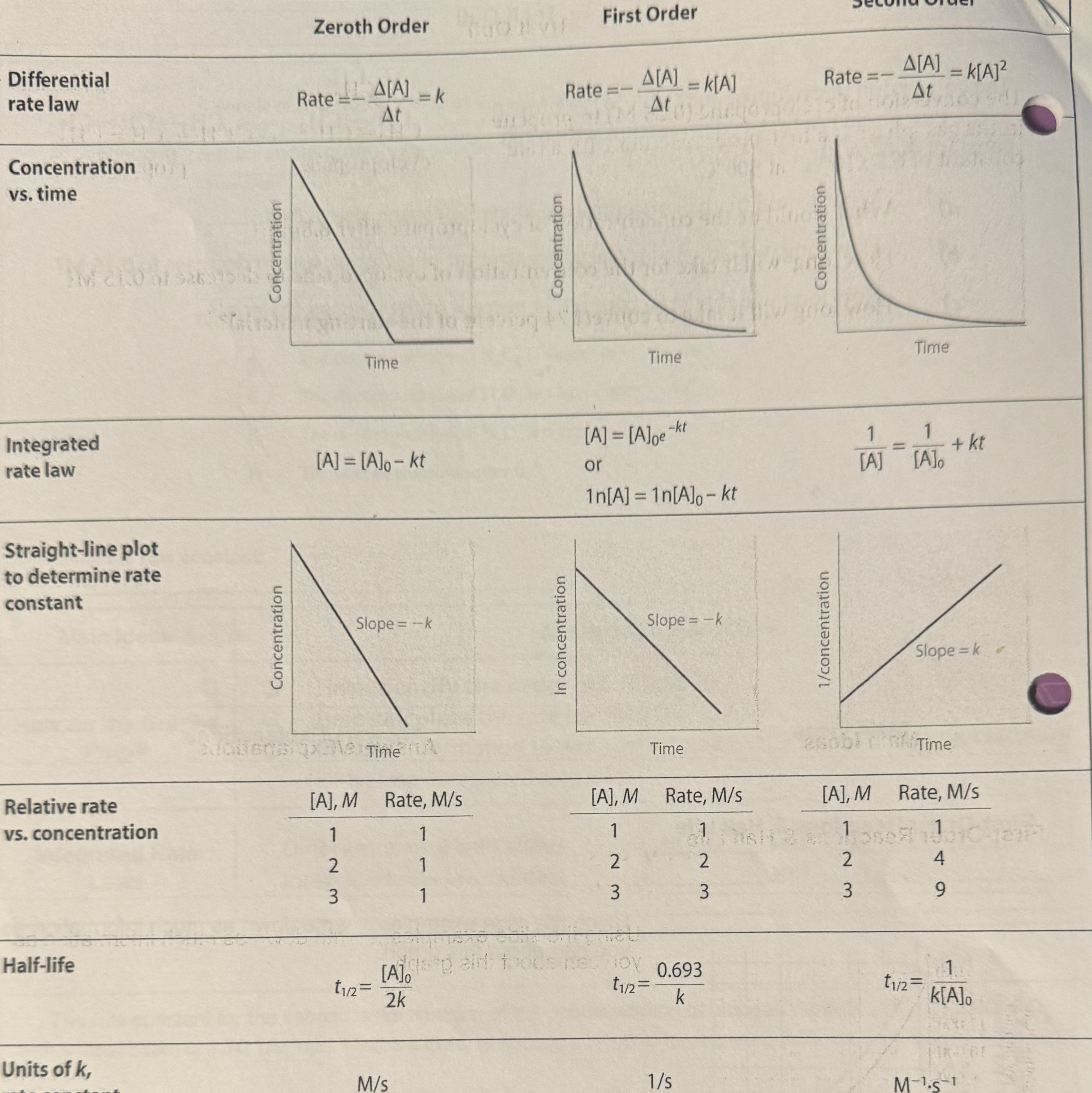
5.3 Concentration Changes Over Time
Determining Rate Law for a Reaction
graph of your data for concentration versus time, make 3 graphs
[A] versus t
ln[A] versus t
1/[A] versus t
determine which graph is linear and determine order of reaction
[A] versus t = zero order
ln[A] versus t = first order
1/[A] versus t = second order
determine your constant
zero order: rate = k (k= - slope)
first order: rate = k[A] (k= - slope)
second order: rate = k[A]2 (k = + slope)
Integrated Rate Laws
differential rate laws relates the rate and concentrations
integrated rate law relates amount and time
Cheat Sheet
5.7 / 5.8 Intro to Reaction Mechanism
Reminders
Chemical Reactions
depicts the beginning and end of a reaction
helpful for studying yields and quantities
helps to study change in energy (enthalpy) and probability (entropy)
Collision Theory
for a reaction to occur, a collision must take place with enough energy and in the correct orientation to cause a reaction to take place
Mechanism Overview
reaction mechanism helps address what is happening at the molecular/atomic level and the concentration levels of each species (molecule)
uses suggested elementary steps
arrived at in a semi-empirical method
theoretical that is valid or stems from experiments
Identify Components in Reaction Mechanisms
Catalyst
substance that increases the rate of a reaction by either stabilizing the transition state or forming a new transition state
present at beginning and end
can be in the rate law expression, but cancels out in final balanced equation
Intermediate
temporary substance that is produced and consumed
intermediate will not show up in the final rate law
cancels out in the final balanced equation
Rate Law Mechanism
in a multiple step mechanism, one step will be slower that the others
the rate of a reaction can be no faster than the slowest step
Rate Law and Overall Stoichiometry
cannot use stoic coefficients from balanced equation, overall equation to determine the rate
can use stoich coefficients from an elementary reaction to determine the rate
rate is dependent upon actual collisions
rate is dependent upon actual collisions
likelihood of five particles will collide simultaneously with sufficient energy and the correct orientation is minuscule
Elementary Reactions and Their Rate Laws
Molecularity | Elementary Reaction | Rate Law |
Unimoleulcar | A = products | rate = k[A] |
Bimolecular | A + A = products | rate = k[A]2 |
Bimolecular | A + B = products | rate = k[A][B] |
Termolecular | A + A + A = products | rate = k[A]3 |
Termolecular | A + A + B = products | rate = k[A]2[B] |
Termolecular | A + B + C = products | rate = k[A][B][C] |
Unimolecular: consisting of or involving a single molecule
Bimolecular: consisting of or involving two molecules
Termolecular: consisting of or involving three molecules
low probability
5.9 Pre-Equilbrilium Approximation
Intermediates in Rate Laws
intermediates show up as a product and then are consumed
in lab, cannot control concentration of intermediate
catalysts are common in rate law bc we can control concentration
intermediates should not be in the rate law since we can’t control concentration
to get rid of intermediates from a rate law, we can use a fast equilibrium and the mathematical method of substation
Equilibrium Reaction
reversible reactions where rate forward = rate backwards
“dynamic equilibrium”
Steps to Success
write our rate law from slow step
if intermediate is in rate law, continue to step 2
write our the forwards and backward rate for the fast equilibrium step
set the forward and backward rate equal to each other
solve from intermediate
sub intermediate into rate law from slow step