Chemistry 3.4
Demonstrate understanding of thermochemical principles and the properties of particles and substances - External, 5 credits.
Periodic Trends
Electron Configurations
Electrons orbit the nucleus of an atom in subshells. Each subshell can only hold 2 electrons. These subshells then combine to form suborbitals:
s (sharp) orbitals
p (principal) orbitals
d (diffuse) orbitals
f (faint) orbitals
These are filled in order of increasing energy:
Each energy level contains 1 s orbital with a maximum of one pair of electrons.
The second level has up to 3 p orbitals each with a maximum of one pair of electrons (6 in total).
The third level has up to 5 d orbitals each with a maximum of one pair of electrons (10 in total).
Electrons in the same subshell have opposite spin notation.
Hund’s Rule states that the most stable arrangement of electrons in a subshell is the one with the greatest number of parallel spins. If orbitals of the same energy are available, and empty one will be filled first.
Isoelectronic refers to two different chemical species with the same electron configuration. E.g. Kr and Br-.
Rules for filling sublevels and suborbitals:
All orbitals hold a maximum of 2 electrons. An orbital containing 2 electrons is called a filled orbital.
Electrons occupying the same orbital must have opposite spin.
Electrons fill at lowest energy sublevels first.
Hund’s Rule.
The Aufbau Principle states that the ‘1’ shells are written before the ‘2’ shells, etc. Even if the ‘4s’ appears to come before ‘3d’ on the periodic table.
The Noble Gas Shortcut allows us to write [Ar] instead of 1s² 2s² 2p^6 3s² 3p^6 for elements after argon on the periodic table.
The 4s shell will be filled before the 3d shell (although it is written after) except in Chromium[Ar] 3d^5 4s^1 and Copper [Ar]3d^{10} 4s^1due to Hund’s Rule. When positive ions form, electrons will be removed from the furthest shell available (4s).
Atomic and Ionic Radii
The radius of an atom can be measured by forcing two atoms to covalently bond. The radius is half the distance between the nuclei. This is measured in nanometres (nm or \times{10^{-9}} metres). This also assumed that the atoms are spherical. The radius will:
Increase down each group → as electrons are in higher energy levels away from the nucleus;
Decrease across each period → as the atomic number increases and there is greater attraction between the nucleus and electrons.
E.g. Lithium has a radius of 0.123nm, Fluorine has a radius of 0.072nm and Potassium has a radius of 0.203nm
We cannot technically measure the radius of noble gases, as they cannot be forced to bond together.
For ions, the radius will:
Increase down each group → as electrons are in higher energy levels away from the nucleus;
Increase across each period → as electrons go from being removed (left-hand side) to being added (right-hand side), so there are more electrons and the radius increases.
Ionisation Energies
The ionisation energy is the amount of energy required to remove one electron from one mole of an atom in the gaseous state, producing one mole of gaseous ions with a charge of 1+. It is measured in kJmol^{-1}.
E.g. the first ionisation energies of Chlorine and Sodium are shown in the equations:
Cl(g)→Cl^+(g)+e^- \Delta{H}=1251.1kJmol^{-1}
Na(g)→Na^+(g)+e^- \Delta{H}=494kJmol^{-1}
The ionisation energy tells us the ease with which electrons are removed from an atom. By successively removing electrons, we can deduce the electron configuration. The factors that can influence ionisation energy are:
The size of the atom;
The charge on the nucleus;
The screening effect of inner energy levels;
The type of electron involved. Due to the shape of its orbital, an s electron is more tightly held than a p or d electron, so is harder to remove.
In general, ionisation energy will decrease down each group and increase across each period. However, this change is not regular due to the different properties of s, p, and d electrons.
Electronegativity
Electronegativity is used to predict the degree of polarity or ionic character of a bond. It is a measure of the attraction between a nucleus and a bonded pair of electrons. The value of electronegativity is shown on a Pauling Scale (no units). Fluorine is the most electronegative element (value of 4.0), and Caesium is the least electronegative (value of 0.7).
The type of bonding present can be roughly estimated using the difference in electronegativity between the two bonded species.
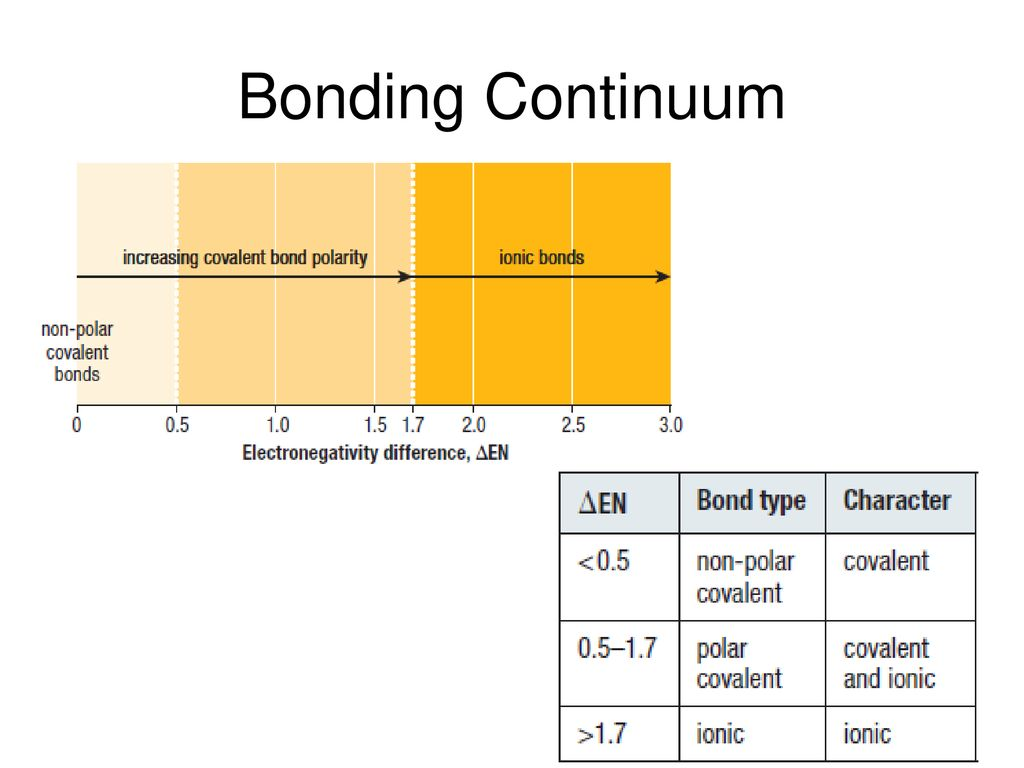 Electronegativity decreases down each group and increases across each period.
Electronegativity decreases down each group and increases across each period.
Properties of Particles and Substances
Lewis Structures for Level 3
Octet Expansion is when atoms with an atomic number greater than 14 (silicon) can hold more than 4 pairs of electrons in their valence shell, using empty d orbitals.
![]() There are 13 molecular shapes at NCEA Level 3:
There are 13 molecular shapes at NCEA Level 3:
![]()
Polarity
When there is a bond between two atoms of unequal electronegativity, there is a permanent dipole (charge), symbol \delta. This means that one part of the bond will have more negative charge than another. This is referred to as a polar bond. In a molecule with more than two atoms, there may be several polar bonds. The dipole forces of these bonds may either enhance each other or cancel out, depending on the symmetry of the molecule. For example, the molecule CH_3Br is polar, due to a higher concentration of negative charge around the bromine atom, while CH_4 is considered to be non-polar, due to the equal and symmetrical distribution of charge around the molecule. This is said to have no dipole moment.
Molecules themselves are bonded by covalent bonds (e.g. the two N atoms in N_2 gas). However, to make a molecular solid, there must be bonding between each individual molecule. All molecules, whether polar or not, have weak intermolecular forces between them. There are three types of intermolecular force:
Temporary dipole-dipole attractions occur in all molecules. These are also called instantaneous dipole induced forces, as they are short lived while electrons move around the valence shells. The larger the molecule, the stronger the attraction
Permanent dipole-dipole attractions occur in all polar molecules. This is because they have permanent dipoles at opposite ends. The more polar the molecule, the stronger the attraction. Shape affects the attraction as well; a tighter fit leads to stronger attractive forces.
Hydrogen bonding occurs in molecules where H is bonded directly to a highly electronegative atom (F, O, or N). The electronegative atoms attract the one valence electron of H so strongly that the remaining proton on H exerts a strong positive force.
Hydrogen bonds are around 10x stronger than a permanent dipole-dipole bond, and 100x stronger than a temporary dipole-dipole bond. But only 10% the strength of a covalent bond.
Solubility describes different substances’ ability to dissolve in different solvents.
Non-polar substances can only be dissolved in non-polar solvents. This is because they are attracted to each other by their weak intermolecular forces. The forces may be disrupted but new ones will form. As water is polar, this means that non-polar substances are insoluble in water. This is because they are unable to form hydrogen bonds with the water molecules and break apart from the rest of the molecules.
Polar substances can only be dissolved in polar solvents, like water, as they are able to form hydrogen bonds, or break the hydrogen bonds in water and form new bonds in the products. They cannot be dissolved in non-polar solvents. Bonding and Intermolecular ForcesAttractive Forces and Properties
Thermochemistry
Phase Changes
Heat vs. Temperature: Heat is the sum of the kinetic energies of all the particles in a sample. Temperature is proportional to the average kinetic energy of the particles.
E.g. if a spark falls it has high kinetic energy, but few particles so it won’t do much damage.
Specific Heat Capacity (c or s) is the amount of heat energy required to raise 1g of the substance by 1K
Absolute Temperature is measured in Kelvin (K). At 0K, the kinetic energy of particles is zero.
Phase Change Diagram
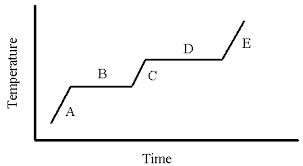 A → Solid, increasing temperature. Particle vibrations increasing. Bonds holding particles together in a rigid structure are broken.
A → Solid, increasing temperature. Particle vibrations increasing. Bonds holding particles together in a rigid structure are broken.
B → Solid melts into a liquid (fusing)
C → Liquid, increasing temperature. Particle vibrations increasing. Bonds between particles break, allowing escape.
D → Liquid evaporates into a gas (vapourisation)
E → Gas, increasing temperature.
Enthalpy Changes
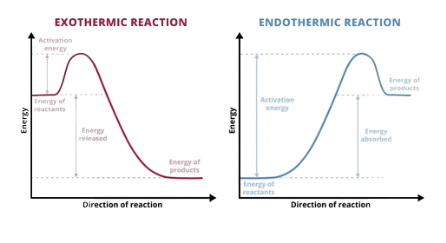 \Delta_{fus}H → latent heat of fusing (melting or fusing) is the energy needed to change 1mol of substance from solid to liquid (or vise-versa) at the melting point.
\Delta_{fus}H → latent heat of fusing (melting or fusing) is the energy needed to change 1mol of substance from solid to liquid (or vise-versa) at the melting point.
\Delta_{vap}H → latent heat of vapourisation (evaporating or condensing) is the energy needed to change 1mol of substance from liquid to gas (or vise-versa), at the boiling point.
\Delta_{sub}H → latent heat of sublimation (deposition or sublimation) is the energy needed to change 1mol of substance from solid to gas (or vise-versa).
\Delta_{fus}H will always be less than \Delta_{vap}H.
\Delta_cH^o → heat of combustion is the standard enthalpy change when 1mol of substance is completely burned. All reactants and products in standard states. Combustion requires oxygen to occur.
E.g. combustion of ethylene: C_2H_4(g)+3\frac{1}{2}O_2(g)→2CO_2(g)+3H_2O(l)
\Delta_fH^o → heat of formation is the standard enthalpy change when 1mol of substance is formed from its elements. Reactants and products in standard states.
E.g. formation of ethanol: 2C(s)+3H_2(g)+\frac{1}{2}O_2(g)→C_2H_5OH(l)
Thermochemical Calculations
There are four types of thermochemical calculations that can be used:
Using practical data: \Delta{H}=m\times\Delta{}t\times{c}
Using bond energies: \Delta{H}=\Sigma{broken}-\Sigma{formed}
Using heats of formation: \Delta{H}=\Sigma{products}-\Sigma{reactants} (only if ALL data is \Delta_fH)
Using Hess’s Law: \Delta{H}_1=\Delta{H}_2+\Delta{H}_3
With Hess’s Law, it does not matter how the reaction proceeds, as long as the initial conditions of the reactants and final conditions of the products are met. For example, \Delta_fH data can be used to find the \Delta_cH.
Entropy
Entropy (S) is a measure of the disorder of the system. There are four ways of changing the entropy of the system:
Temperature → Increased temperature leads to increased kinetic energy. This allows the particles to move around more, leading to more possible arrangements of particles in the system and higher entropy.
Number of Particles → The more particles, the more possible arrangements of particles in the system, so the higher the entropy.
Complexity → The more complex the system, the higher the entropy.
Volume → In a gaseous system, the more volume the system has, the higher the entropy.
Useful Formulae
n=\frac{m}{M}
n=cV
q=mc \Delta T
\Delta_r H^o=\frac{-q}{n}
\Delta_r H^o=\Sigma \Delta_f H^o (products) - \Sigma \Delta_f H^o (reactants)
Practice Questions
Q: Explain why a chlorine atom has a greater first ionisation energy than a sodium atom.
A: Sodium and chlorine both have 3 electron shells, but as chlorine has a higher atomic number (more protons in the nucleus) than sodium, it has a stronger attraction between its nucleus and electrons. Therefore, it takes more energy to ionise chlorine than sodium.
Q: Which atom has the lowest first ionisation energy, chlorine or iodine? Explain your choice.
A: Iodine. Both chlorine and iodine have 7 valence electrons, but iodine in the fifth period has more electron shells than chlorine in the third period. The larger atomic radius and increased shielding effect from the additional electron shells in the iodine atom mean it requires less energy to ionise than chlorine.
Q: Explain why arsenic and beryllium both have larger atomic radii than nitrogen.
A: Atomic radius increases down each group and decreases long each period. Beryllium has a smaller atomic radius than nitrogen as nitrogen has a larger atomic number, meaning it has a greater positive charge pulling its electrons closer to the nucleus. As both beryllium and nitrogen have 2 electron shells, this means that nitrogen has the smaller radius. Arsenic is in the fourth period, which means it has more electrons shells and more protons than nitrogen. As there are more electron shells between the valence shell and the nucleus with increased positive charge, there is less attraction between them, so the atomic radius of arsenic is larger than nitrogen.
Q: Why does a potassium ion (K^+) have a smaller radius than a potassium atom?
A: Potassium forms a positive cation by donating electrons. This means that it loses its outermost shell, decreasing its radius.
Q: Why does a sulfide ion (S^{2-}) have a larger radius than a sulphur atom?
A: Sulphur forms a negative anion by receiving electrons to fill its valence shell. As more electrons are added, the repulsion between them increases, pushing them further apart. This increases the radius.
Q: Give the electron configurations of:
(a) Sulphur atom
(b) Chromium atom
(c) Potassium ion
(d) Selenium atom
(e) Copper atom
(f) Sulfide ion
A:
(a) 1s²2s²2p^63s²3p^4
(b) 1s²2s²2p^63s²3p^63d^54s^1
(c) 1s²2s²2p^63s²3p^6
(d) 1s²2s²2p^63s²3p^63d^{10}4s²4p^4
(e) 1s²2s²2p^63s²3p^63d^{10}4s^1
(f) 1s²2s²2p^63s²3p^6
Q: What shape is ClF_3? Justify your answer.
A: T-shaped. The chlorine atom in the centre has five potential bonding areas around it, of which three are occupied by fluorine atoms. This give two lone pairs. Valence shell electron pair repulsion theory states that each electron pair around the central atom will exert maximum repulsion to give maximum separation between sets. The lone pairs will push the three bonded sets to one side of the molecule, giving a T-shape.
Q: Justify the shape of IF_4^+.
A: The iodine atom in the centre has five potential bonding areas around it. Four of these are occupied by fluorine atoms, leaving one lone pair. This pushes the four bonded sets to one side of the molecule due to VSEPR theory, which minimises repulsive forces by pushing electron sets to their maximum distances from one another. With four bonded areas and one lone pair, this is a see-saw shape.
Q: Is the C=O bond polar or non-polar? Explain.
A: Polar. As oxygen is slightly more electronegative than carbon, the bonded electrons are more attracted to it, giving a negative dipole on the oxygen side of the molecule.
Q: C-Cl bonds are polar. Explain why CCl_4 is a non-polar molecule.
A: Carbon has four bonding areas around it, and all are bonded to chlorine atoms. This gives a tetrahedral shape, which evenly distributes the dipoles due to VSEPR theory. This means that the molecule has no overall charge at any point and is non-polar.
Q: Describe the shape and polarity of the BrF_5 molecule.
A: The central bromine atom has six potential bonding areas around it, of which five are filled by fluorine atoms. This leave a lone pair, giving a square pyramidal shape. Due to the lone pair and VSEPR theory, the Br-F bonds are not evenly distributed. As these are polar bonds (F has greater electronegativity than Br), there is a negative dipole induced on the side of the molecule with the F-Br bonds and a positive dipole near the lone pair. Therefore, this is a polar molecule.
Q: Describe the intermolecular forces present between these molecules:
(a) HCl
(b) HF
(c) CH_4
A:
(a) This is a polar molecule, so both permanent and temporary dipole-dipole attractions are present. No hydrogen bonding is present.
(b) This is a polar molecule, and with the H-F bond, hydrogen bonding is present in addition to temporary and permanent dipole-dipole attractions.
(c) This is a non-polar molecule, so only temporary dipole-dipole attractions are present.
Q: Explain the difference in boiling points between HF and HCl.
A: HF is polar and has strong hydrogen bonds between molecules. Meanwhile, HCl has only permanent and temporary dipole-dipole attractions. This means that a lot more energy is required to break apart the molecules of HF than HCl, so it has a higher boiling point.
Q: Which molecule, C_3H_8 or C_4H_{10}, has a higher boiling point? Justify.
A: Both molecules are non-polar, with weak intermolecular forces due to temporary dipole-dipole attractions. However, As C_4H_{10} has a higher molar mass, it will require more energy to boil, so it will have a higher boiling point.
Q: Does C_4H_{10} or CH(CH_3)_3 have a higher boiling point? Justify.
A: These are both non-polar molecules with the same molar mass. However, C_4H_{10} is a straight-chain molecule, while CH(CH_3)_3 is a branched-chain molecule. Straight-chain molecules pack together more tightly, which increases the energy required to break them apart. This means that C_4H_{10} will have a higher boiling point.
Q: Predict the entropy change of solid MgCl_2 dissolving into its ions.
A: The solid, rigid structure is separated and the particles disperse throughout the solution. This means that there is more disorder and entropy increases.
Q: Predict the entropy change of H_2O(g)→H_2O(l)
A: Gaseous particles that were in constant random motion, become slower, more ordered liquid particles. Temperature and disorder decrease, so entropy decreases.