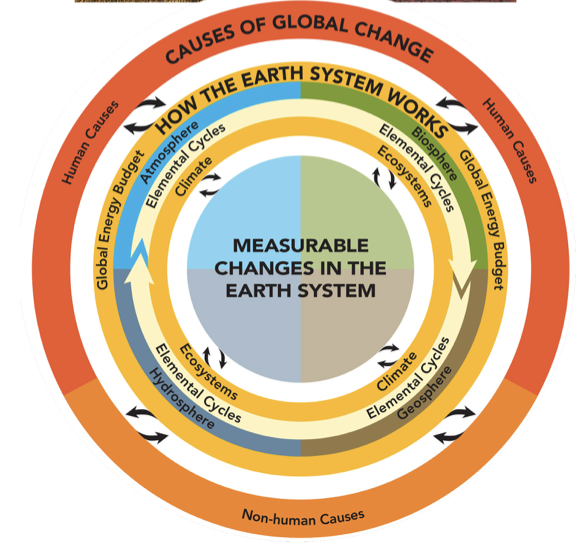
4.3 Cycles of Matter >
The building blocks of life (Water, Carbohydrates, lipids, nucleic acids, and proteins) are essential to life and are primarily made up of oxygen, hydrogen, carbon, nitrogen, phosphorus, and potassium(known as the “essential nutrients”). These six elements are required by living things and are used in large quantities, so why do they never seem to run out? The answer to this is that nature actually recycles its elements between biotic and abiotic factors, and these are referred to as the Biogeochemical cycles(powered by energy flow through the biosphere). The nutrients and matter flowing through ecosystems transform into different compounds because the matter is never created or destroyed.
Processes in Biogeochemical cycles can be categorized into:
biological*(Biosphere-driven by living organisms: photosynthesis, respiration)*
geological(Geosphere-Volcanic eruptions, the forming/breakdown of rock, major underground movement)
physical/chemical*(*Hydrosphere, atmosphere, geosphere-cloud formation, precipitation, the flow of running water, lightning)
human-driven processes*(*Outer Ring-Human activities: mining/burning fossil fuels, clearing land for agriculture, burning/replanting forests, usage of fertilizer).
The Water Cycle
Transpiration vs. Evaporation
Transpiration is when water travels through the biosphere. This happens when water seeps into the soil(Infiltration, the movement of water into soil), and is then taken up by the roots of plants. Excess water that the plant does not need then re-enters the atmosphere through transpiration, or when water evaporates from the leaves of plants.
Evaporation occurs when surface water absorbs heat energy and changes into a gas.
Precipitation
Precipitation is when water falls to the surface, and can happen in many forms: rain, snow, sleet, or hail. ----
☆Precipitation that flows on land into bodies of water is called runoff
☆Water vapor can be carried through the atmosphere by winds before it condenses
☆Rainwater from precipitation may cause erosion, allowing nutrients to be transported
Water storage
97% of water is in the oceans
Water can be stored in aquifers which are groundwater storage areas
Atmospheric Circulation
Heat is transported around the surface of the earth, affecting the water cycle and its condensation/precipitation
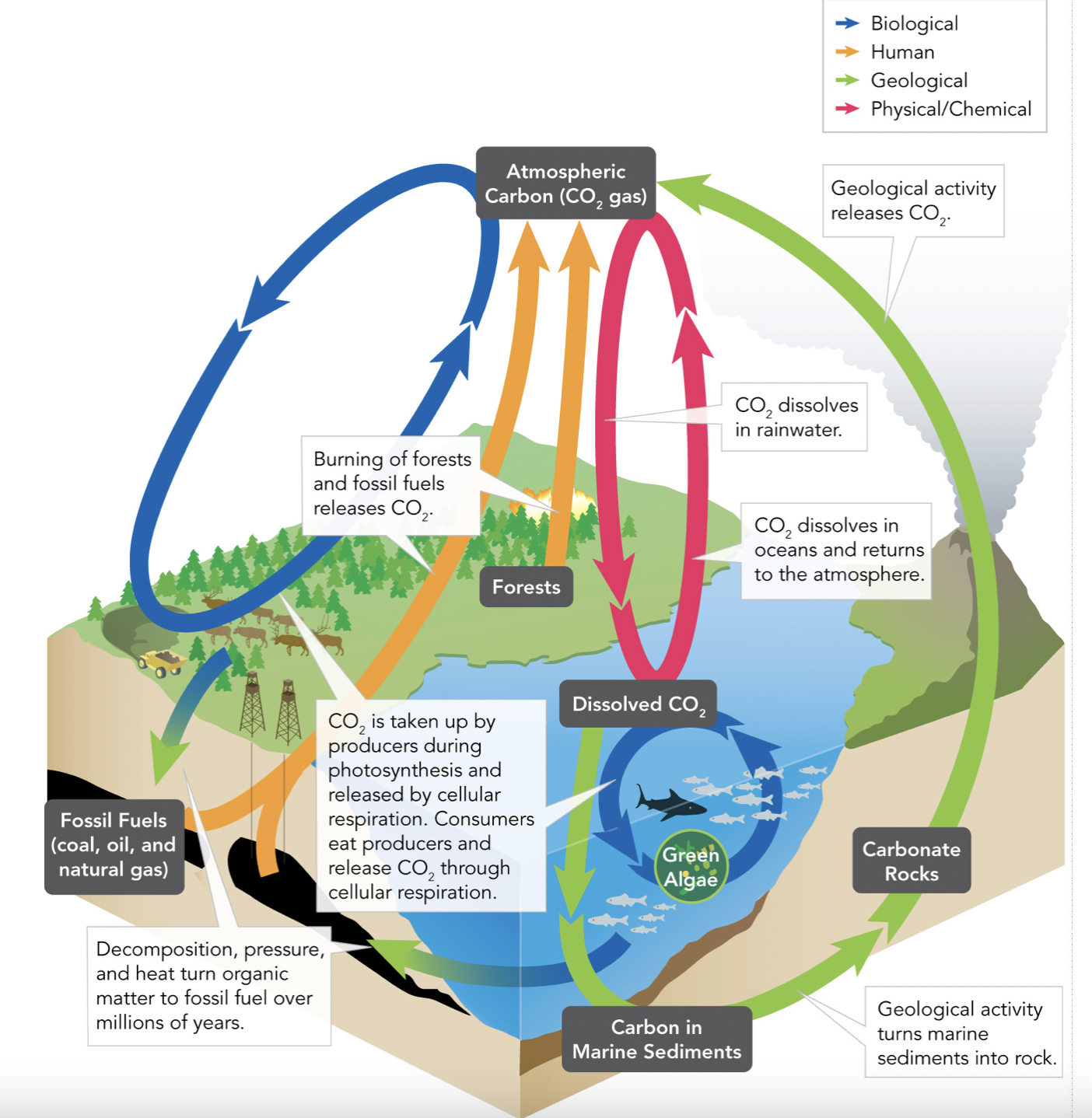
The Carbon Cycle
“Carbon Based Life”: The importance of Carbon
Carbon is a major component of the four building blocks of life, and therefore an essential element to living beings. It contributes to the skeletons of animals, makes up many rocks, and also makes up fossil fuels. Carbon dioxide gas is made up of oxygen and carbon, which play a big factor in retaining heat within the atmosphere.
Processes
The carbon cycle starts biological and ends geological since when living beings die, the organic matter is solidified in the ground, and then turned into fossil fuels.
Adds Carbon
☆Respiration (plants use up the food made from photosynthesis and turn them into energy)
☆Combustion (burning forests)
☆Cellular Respiration (Consumers eat producers and release CO2)
☆Volcanic Activity (Carbon deep under the earth is forced out by the heat of volcanic eruptions)
☆Death of Organisms (decomposers break down organic matter, releasing CO2)
☆Human Activity (ties in with combustion, when we burn natural gases)
Removes Carbon
☆Photosynthesis (plants take CO2 to create glucose)
☆Dissolved carbon dioxide combines with other minerals which settle at the bottom of the ocean. Over time, these carbonates build up and harden into sedimentary rock
The Nitrogen Cycle
Nitrogen is required for proteins and nucleic acids. The majority of nitrogen is found in the atmosphere and is 78% of the air we breathe. The substances ammonia, nitrate ions, and nitrite ions contain nitrogen and are found in the biosphere/geosphere. The hydrosphere also contains significant amounts of dissolved nitrogen.
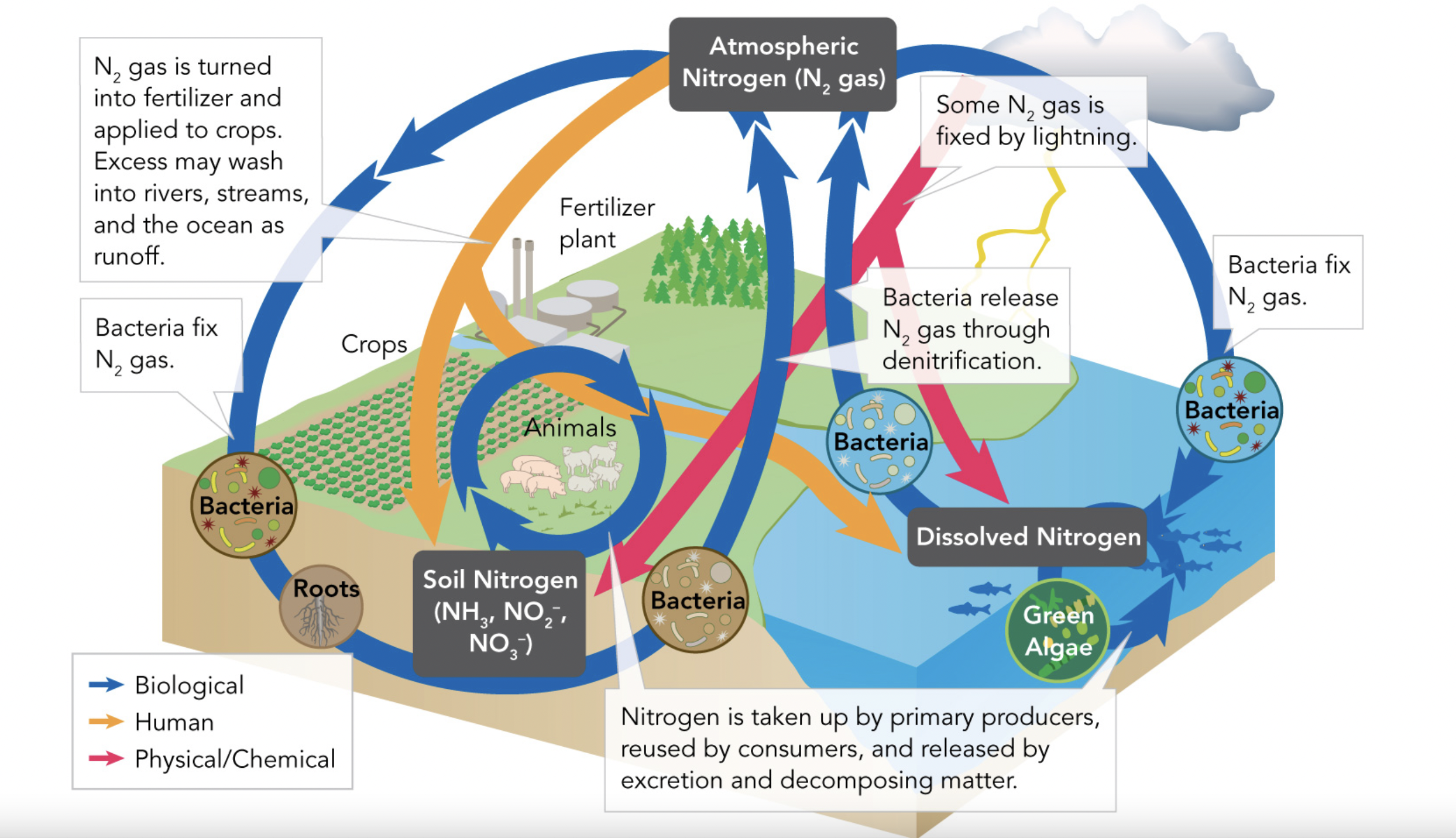
Although there is plenty of nitrogen gas in our earth's system, many living organisms are unable to use it. It only becomes usable when a type of bacteria or lightning converts/fixes nitrogen gas into ammonia. This is called nitrogen fixation, or atmospheric nitrogen fixation when it is done by lightning. Bacteria can also convert ammonia into nitrite/nitrate, which is usable by autotrophs. After this, consumers can eat the primary producers with nitrogen compounds, in order to reuse them. Nitrogen compounds may also be released from dead organisms, which the autotrophs can consume once again. Denitrification is the opposite of nitrogen fixation and is when bacteria turn nitrates into nitrogen gas in order to gain energy.
N2- nitrogen gas, NO3- nitrate, NO2- nitrite, NH3- ammonia
Other:Assimilation is when inorganic (unusable) nitrogen compounds are turned organic, or usable
Summary:
Bacteria/lightning converts nitrogen gas to ammonia => Bacteria converts ammonia to nitrite or nitrate => Primary producers use the nitrite/nitrate => Consumers eat the autotrophs => consumers are decomposed, releasing nitrogen compounds (by fungi/bacteria) => Primary producers take up the nitrogen compounds => cycle restarts
OR
Bacteria/lightning converts nitrogen gas to ammonia => Bacteria converts ammonia to nitrite or nitrate => Other bacteria convert nitrates to nitrogen gas => nitrogen gas is released back into the atmosphere
[Bacteria, lightning, and industry (fertilizer plants) convert nitrogen in the air to ammonia]
Fertilizer
Nitrogen gas is converted into fertilizer for crops(fixes LOTs of nitrogen gas), which may get washed into bodies of water
The Phosphorous Cycle
Phosphorous is a major component of DNA and RNA but it does not have a gaseous phase like the other three elements, making it unable to cycle through the atmosphere.
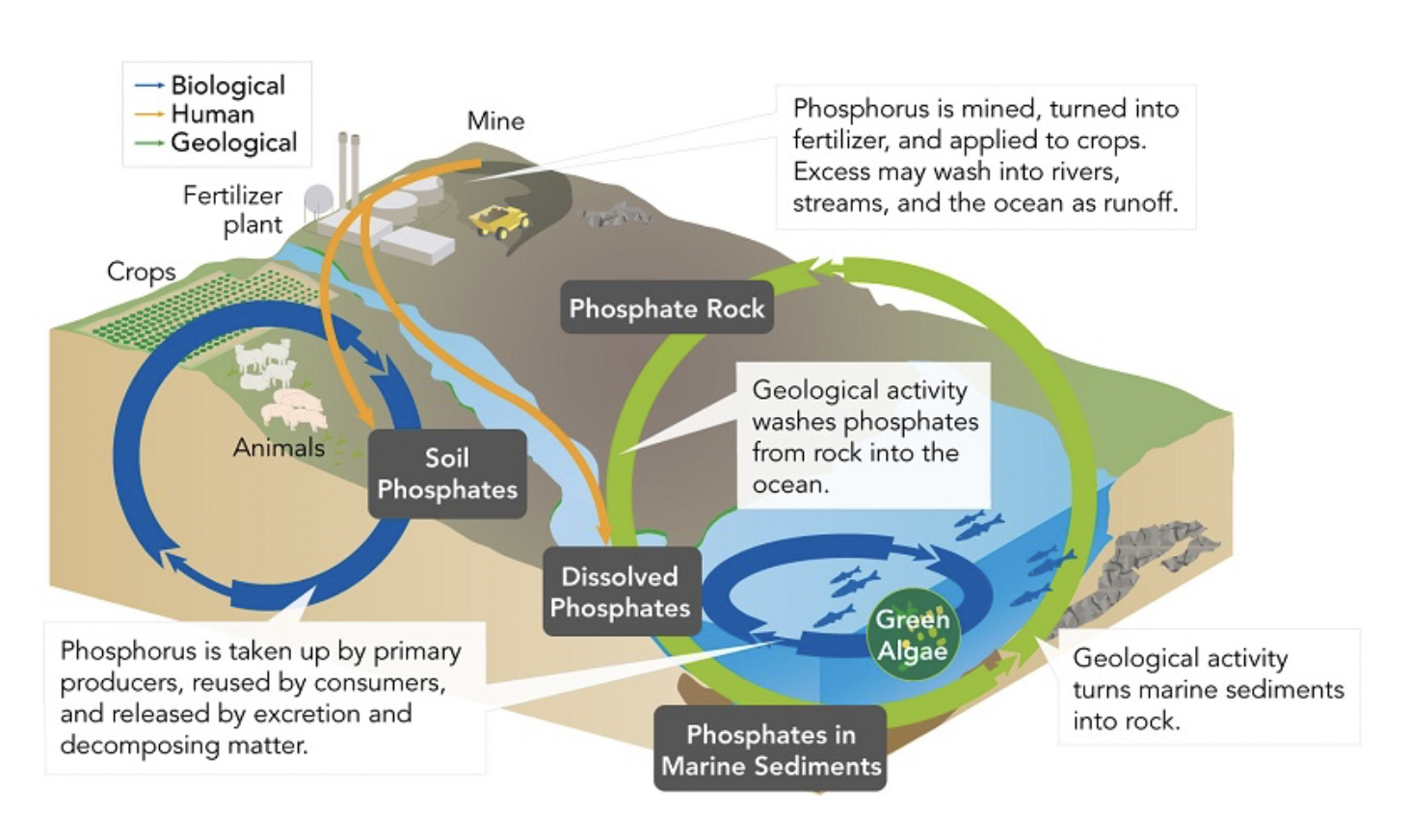
Main Reservoir: Rocks/Soil
☆Phosphorus can be turned into fertilizer, just like nitrogen gases, and the excess may run off into bodies of water (Human activity)
☆Phosphorus travels through the trophic level and, and is continuously used in a cycle
(Absorbed by plants, consumed by animals, Organisms die which returns phosphorus to soil/aquatic ecosystem waste)
☆Geological movements are involved in the transportation of phosphates, as well as the conversion of sediments (Erosion from rocks, water rushes over rock => dissolved phosphorus and washes it into rivers and streams)
Other things to note
How does Oxygen factor into these cycles?
This element takes part in all of the Biogeochemical cycles by going through the whole process along with the other three elements carbon, nitrogen, and phosphorous.
What’s the equation for photosynthesis?
6CO2 + 6H2O → C6H12O6 + 6O2
Vocabulary
Biogeochemical Cycle- The nutrients and matter flowing through ecosystems transform into different compounds because the matter is never created or destroyed.
Nutrient- a substance that organisms require to live and grow (helps organisms build tissue/carry out necessary life processes)
Nitrogen Fixation- the conversion of nitrogen gas to a usable form
Denitrification- the process of bacteria converting nitrates to nitrogen gas
Limiting Nutrient- a nutrient that cycles slowly and limits the growth of an organism, if a single essential nutrient is low, productivity will be limited (In soil, fertilizer is used to maximize the growth of crops; In seawater, nitrogen is usually the limiting nutrient; In freshwater, phosphorous is usually the limiting nutrient)
Primary Productivity- The rate at which autotrophs can produce organic material
eutrophication- when an environment becomes overly enriched with nutrients, algae will excessively grow in the body of water; increased availability of limiting growth factors required for photosynthesis