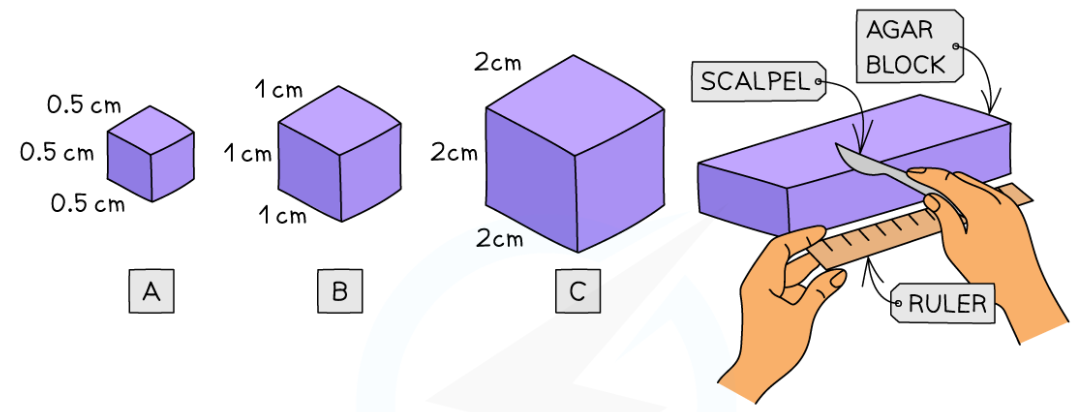
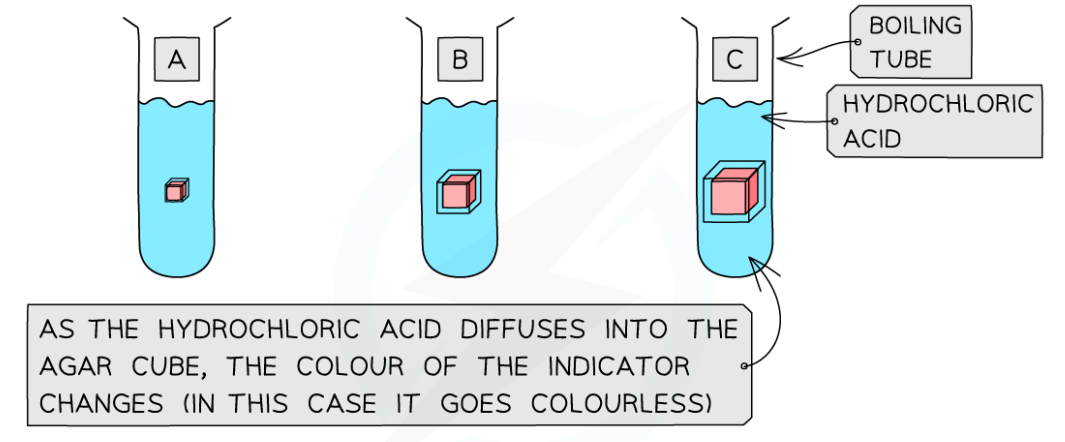
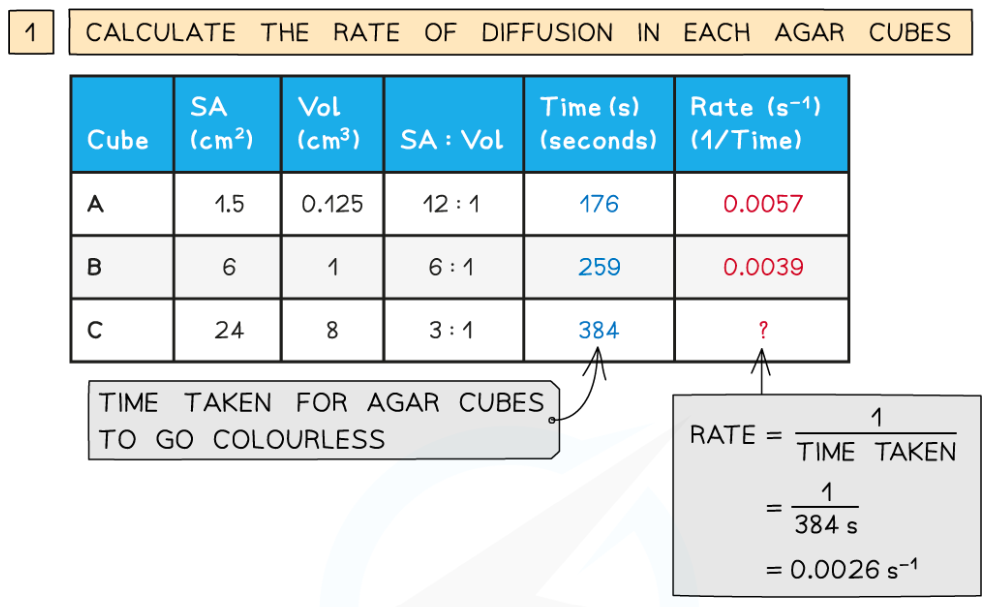
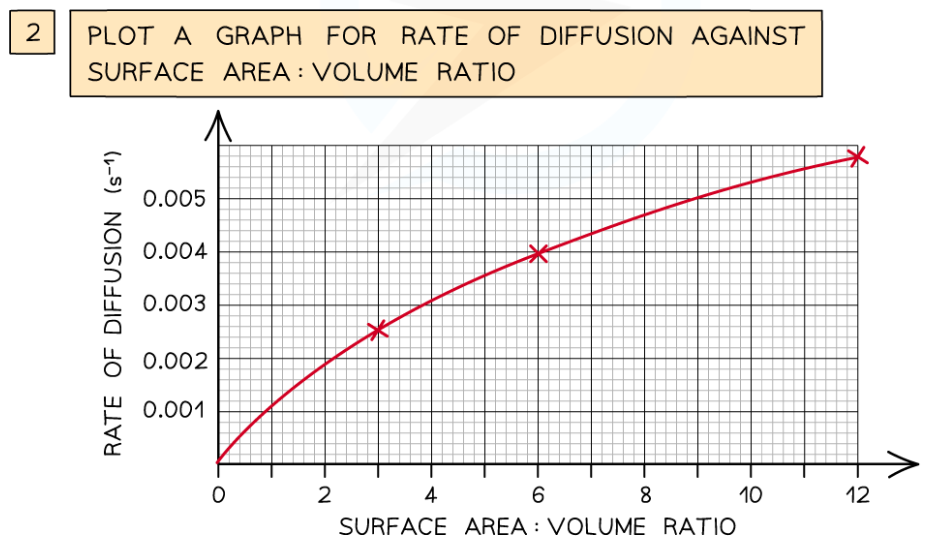
0.0(0)
Chat with Kai
Explore Top Notes Note
Note Studied by 224 people
Studied by 224 people Note
Note Studied by 21 people
Studied by 21 people Note
Note Studied by 13 people
Studied by 13 people Note
Note Studied by 65 people
Studied by 65 people Note
Note Studied by 2 people
Studied by 2 people Note
Note Studied by 4 people
Studied by 4 people
6.2 State Expansion
Factors and Multiples
Chapter 2: The Developing Brain
Princeton Review AP Calculus BC, Chapter 11: Parametric Equations, Polar Coordinates, and Vector-Valued Functions
AFPF casus 6
Middle Childhood: Physical Growth
 Knowt
Knowt