Hypoxia response Part 3 (Testing of Hypothesis)
Hypothesis and Predictions
Hypothesis: The activity of the mitochondrial electron transport chain regulates the degradation of HIF1 alpha by controlling cellular oxygen availability.
First Prediction: Nitric oxide should increase the degradation rate of HIF1 alpha under hypoxic conditions, indicating that it speeds up the degradation process in hypoxia.
Measurement of Protein Degradation
Common Method: Block new synthesis of the protein using an inhibitor, then measure how quickly the protein disappears over time.
Experimental Method
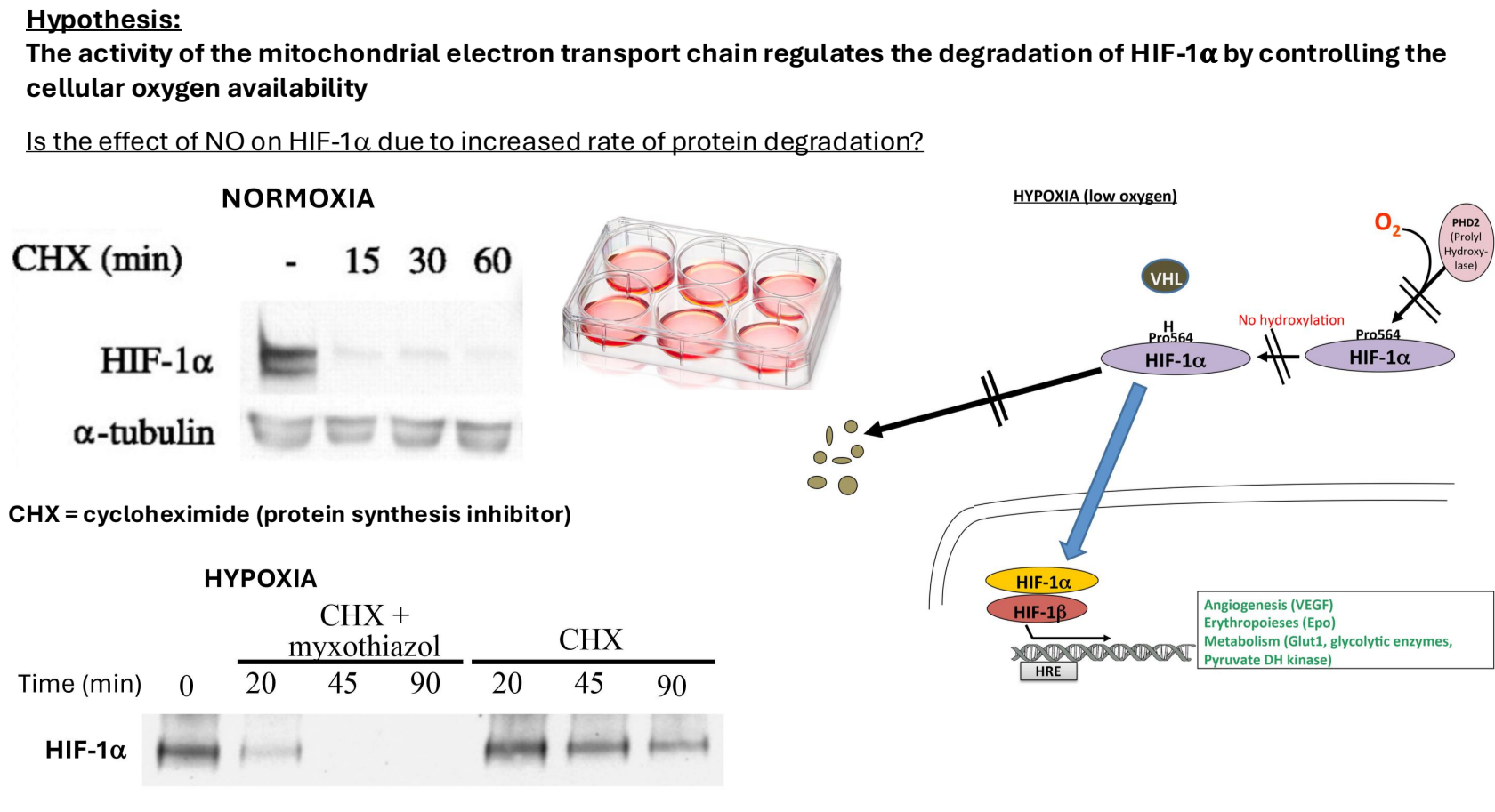
Induction of HIF1 alpha:
Cells are subjected to hypoxia to induce HIF1 alpha.
Cyclohexamide, a protein synthesis inhibitor, is added to block new protein synthesis.
Cells are then lysed at different time points to measure HIF1 alpha levels:
Immediate Lysis: High levels of HIF1 alpha detected.
15 Minutes Post-Lysis: HIF1 alpha is nearly undetectable, indicating rapid degradation (consistent with a half-life of 5 minutes in normoxic conditions).
Degradation Under Hypoxic Conditions
Under hypoxia, HIF1 alpha degrades more slowly due to low oxygen availability affecting hydroxylation and degradation processes.
Addition of mitochondrial complex 3 inhibitor increases HIF1 alpha degradation rate, supporting the hypothesis that mitochondrial activity influences degradation rates.
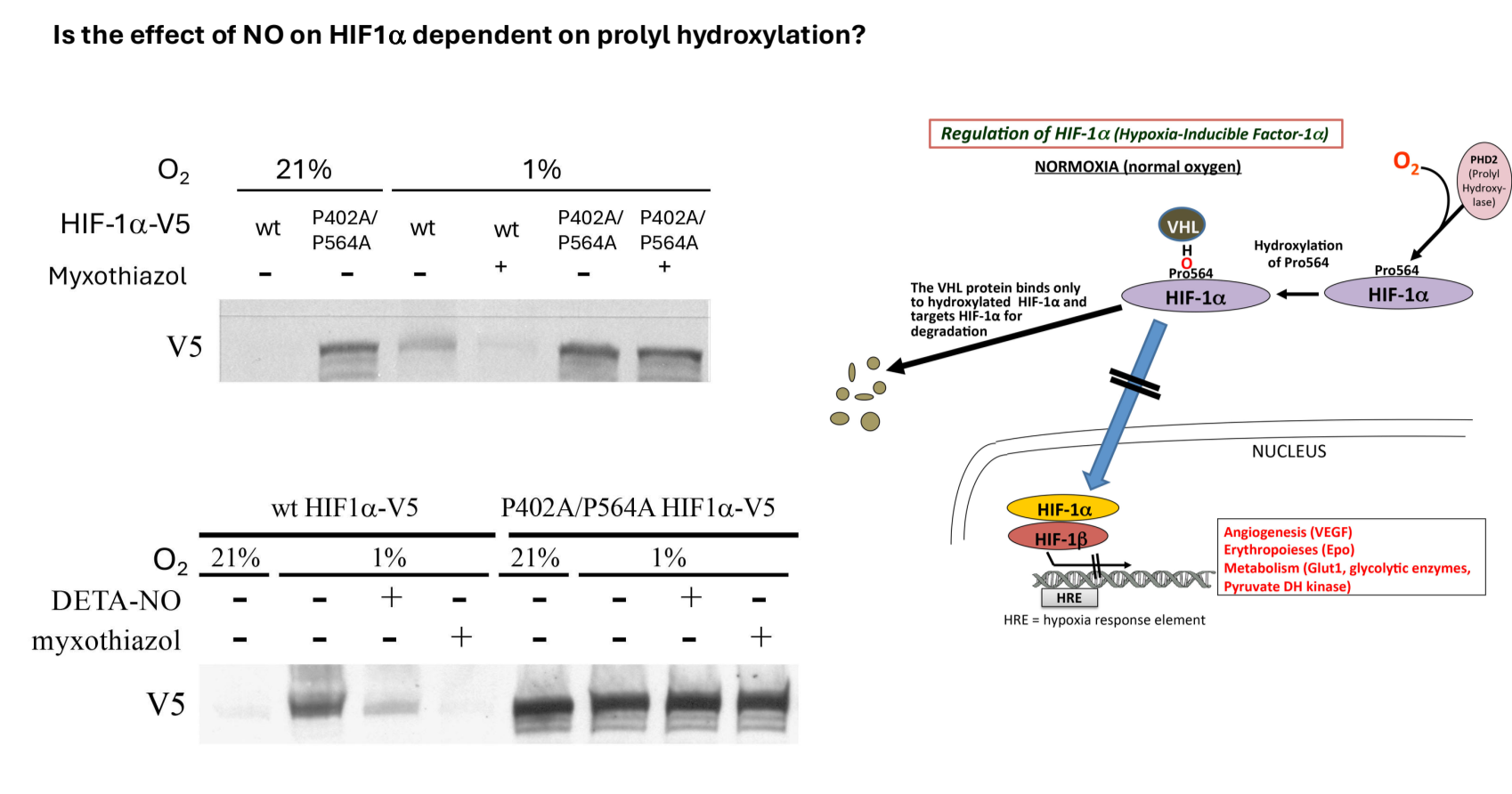
Polyhydroxylation and Protein Binding
Key Residues for Hydroxylation:
Proline 564 and proline 402 are the residues that become hydroxylated and influence binding to VHL (Von Hippel-Lindau protein).
Mutations to alanine were introduced in both residues to study their impact on degradation.
Transfection and Western Blot Analysis
Cells transfected with either wild-type or mutant HIF1 alpha protein.
Under normoxia, wild-type HIF1 alpha is not present; however, it is induced under hypoxia.
Finding: The mutant form accumulates even under normoxic conditions due to inability to be hydroxylated and thus degraded.
In the presence of mitochondrial inhibitors, no change in mutant protein expression occurs under hypoxic conditions.
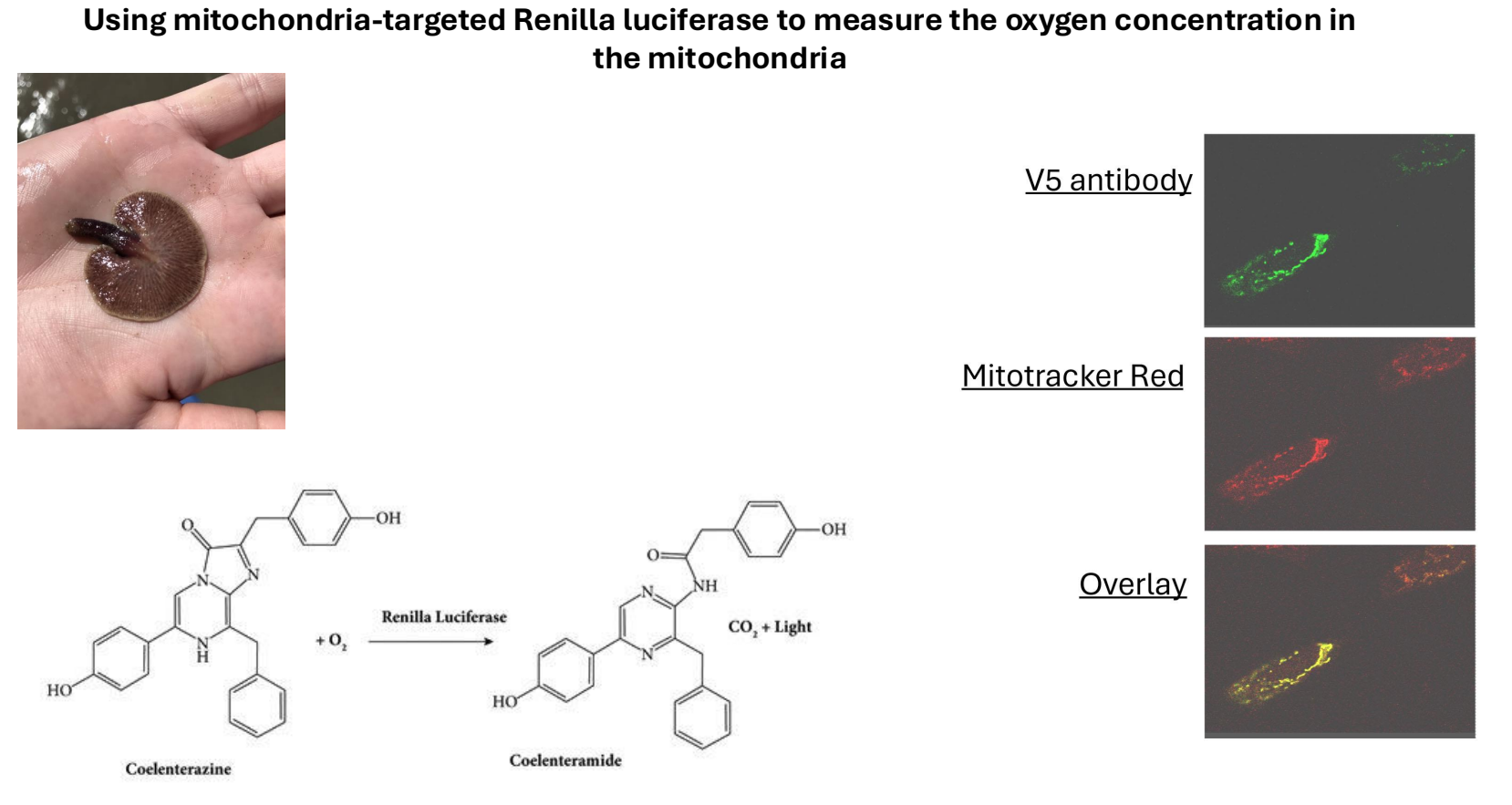
Measuring Intracellular Oxygen Concentration
Challenge: Measure intracellular oxygen concentration, more complex than external measurements (e.g. using oxygen electrodes).
Solution: Use a different luciferase enzyme from Ctenophora vulgaris, which is oxygen-dependent but does not rely on ATP.
A plasmid was generated that targets this luciferase to mitochondria, allowing oxygen sensing within cells.
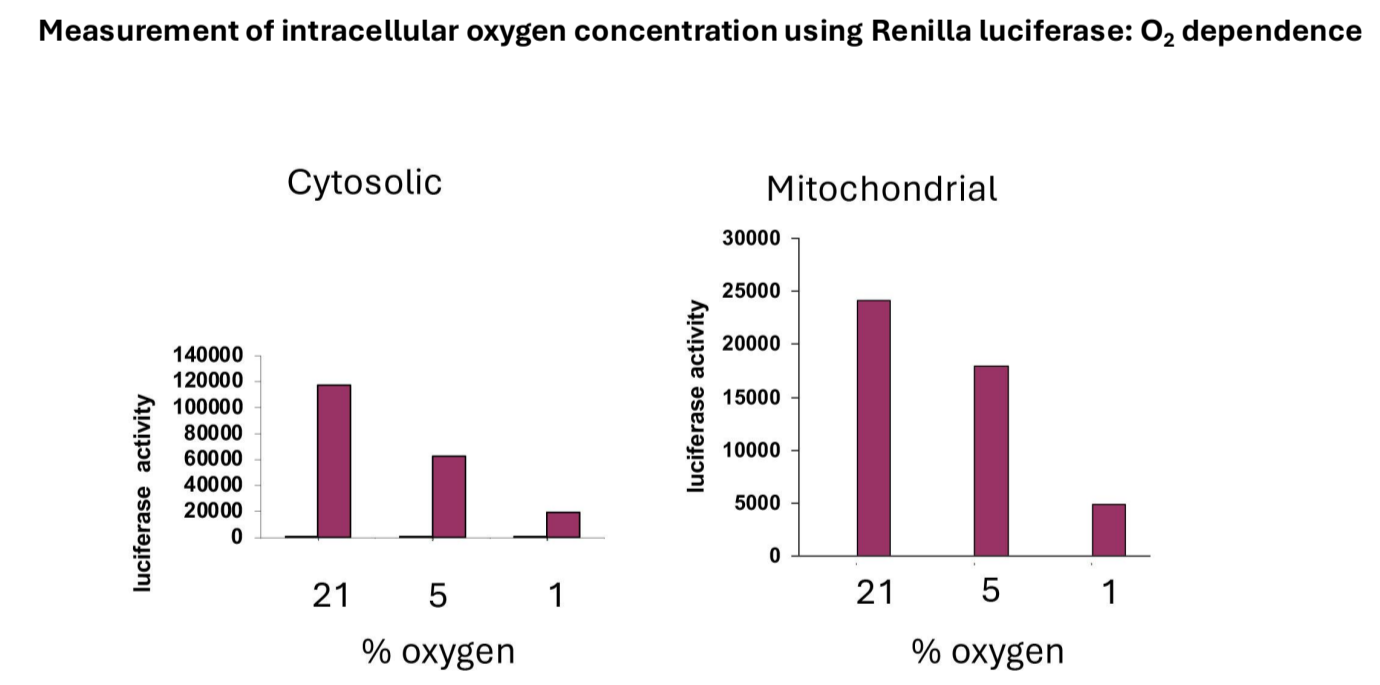
Validation: Confirmed that luciferase activity is dependent on oxygen concentration in both cytosolic and mitochondrial settings, with increased activity at higher oxygen levels.
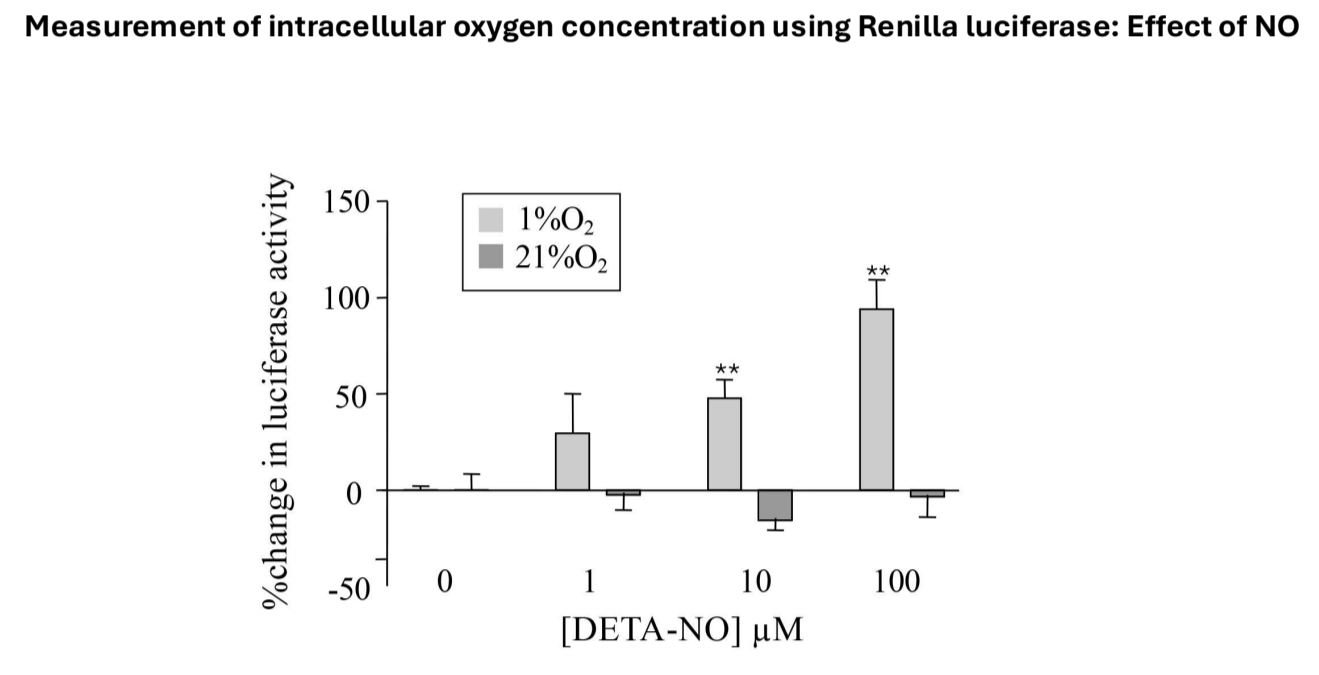
Nitric Oxide's Effect on Oxygen Levels
Tested if nitric oxide increases intracellular oxygen concentration, measuring changes in luciferase activity:
Normoxic Conditions: Minimal effect on luciferase activity with nitric oxide.
Hypoxic Conditions: Significant dose-dependent increase in luciferase activity, suggesting elevated intracellular oxygen levels that induce degradation of HIF1 alpha.
Impact of Mitochondrial Function on Intracellular Oxygen
In Vitro Limitations: Conditions may not replicate in vivo due to diffusion differences in oxygen supply in 3D tissues.
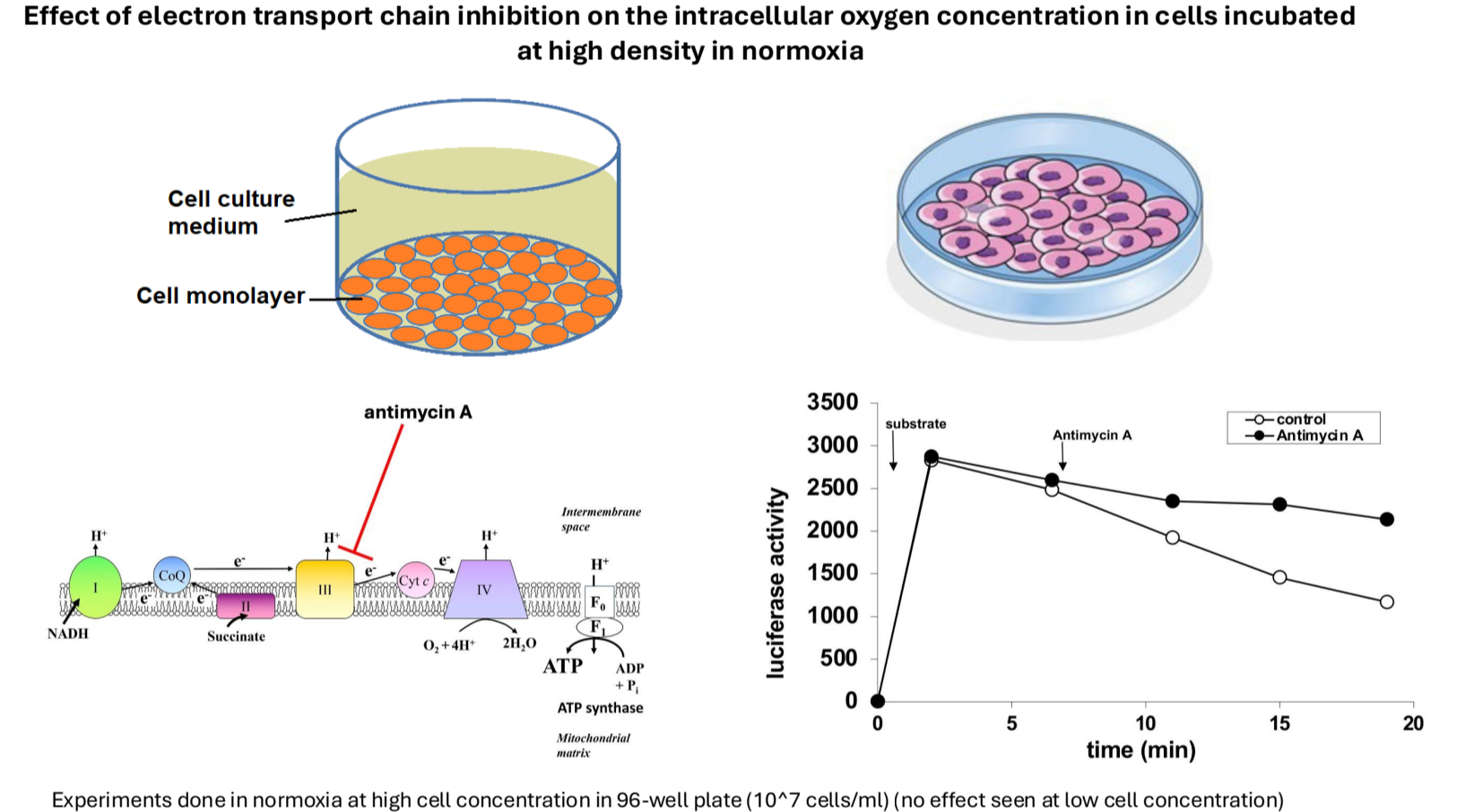
High-Density Cell Cultures: Attempted to mimic in vivo conditions using dense cell populations to study changes in oxygen levels under normoxia and hypoxia.
Findings: Increased cell density leads to decreased intracellular oxygen levels; mitochondrial inhibitors partially prevent this decrease.
HIF1 Alpha Activation Assay
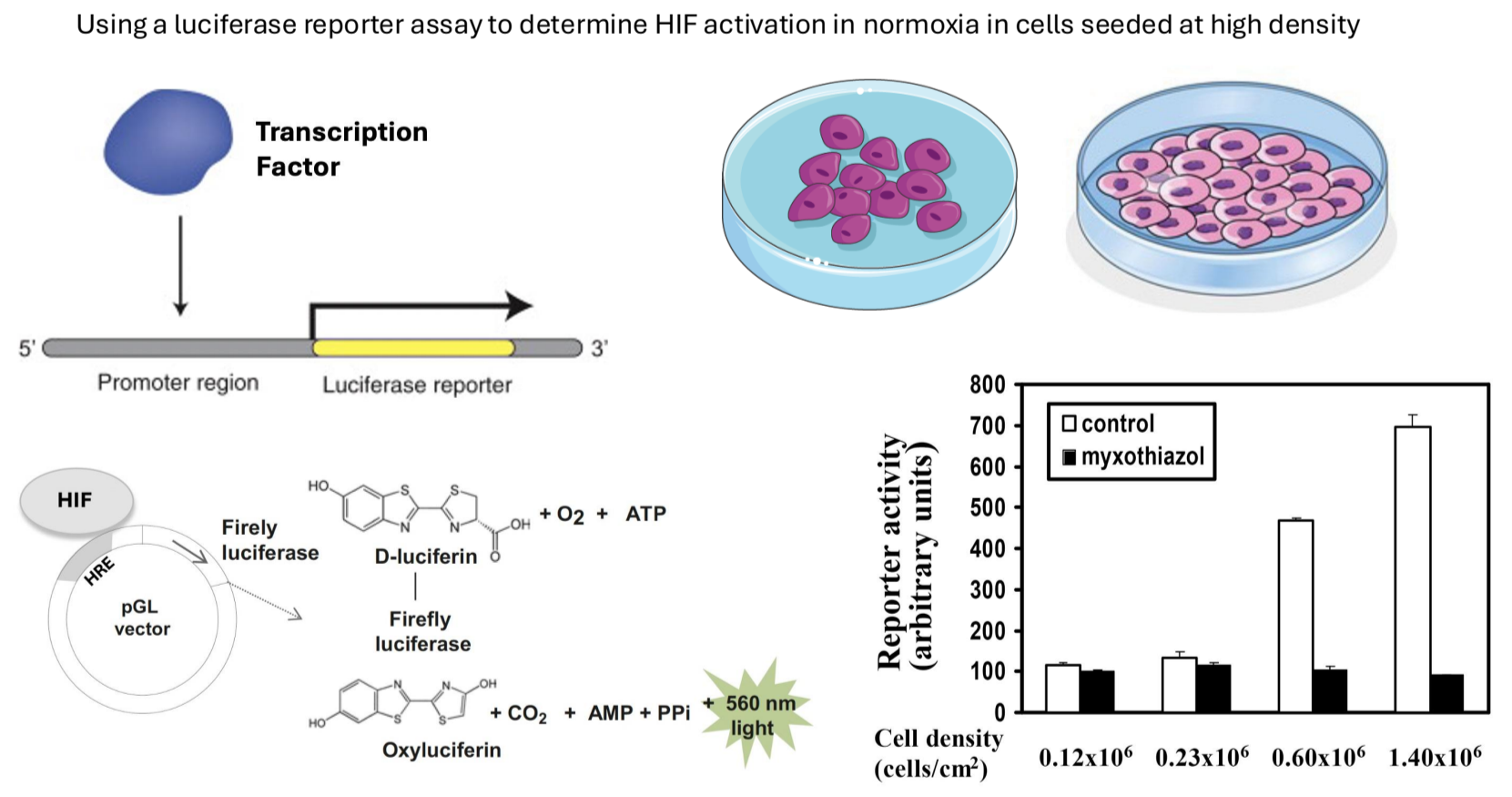
Luciferase Reporter Assay: Used to measure transcriptional activity of HIF1 alpha by cloning its control elements upstream of a firefly luciferase gene.
Higher luciferase activity indicates greater HIF1 alpha transcriptional activation.
Results showed high levels of transcriptional activity were observed under normoxia when cell density was increased, which was blocked by mitochondrial inhibitors, confirming that mitochondria significantly influence intracellular oxygen levels.
Conclusion
Role of Mitochondria: Critical in regulating internal oxygen concentrations, which, in turn, affects HIF1 alpha degradation and activity.
Relevance to Disease: Understanding this mechanism is essential in contexts like tumor biology, where cellular oxygen levels and metabolic processes interact in significant ways.
Warburg Effect: Cancer cells may elevate glycolysis while downregulating oxidative phosphorylation to maintain sufficient intracellular oxygen for essential processes.
 Knowt
Knowt