BIO1011 Week 2
LO1. Determine how organic macromolecules which include proteins, nucleic acids, carbohydrates and lipids, are built from simpler units.
Additional key terms: amino acid, condensation reaction (dehydration reaction), fatty acid, glycosidic bond, monosaccharide, peptide bond, phosphodiester bond, phospholipid, purine, pyrimidine, polypeptide, polysaccharide
● Explain why carbon is the backbone of organic molecules. What type of bonds to carbon atoms form? What other properties does carbon have that enables it to form a diversity of molecules?
Carbon has the ability to form multiple covalent bonds with a wide range of molecules, and is also relatively small compared to other atoms
Carbon can also readily bond to itself, allowing the formation of chains and cyclic molecules
Carbon single bonds can rotate freely, creating different structures in molecules and contributing to their properties
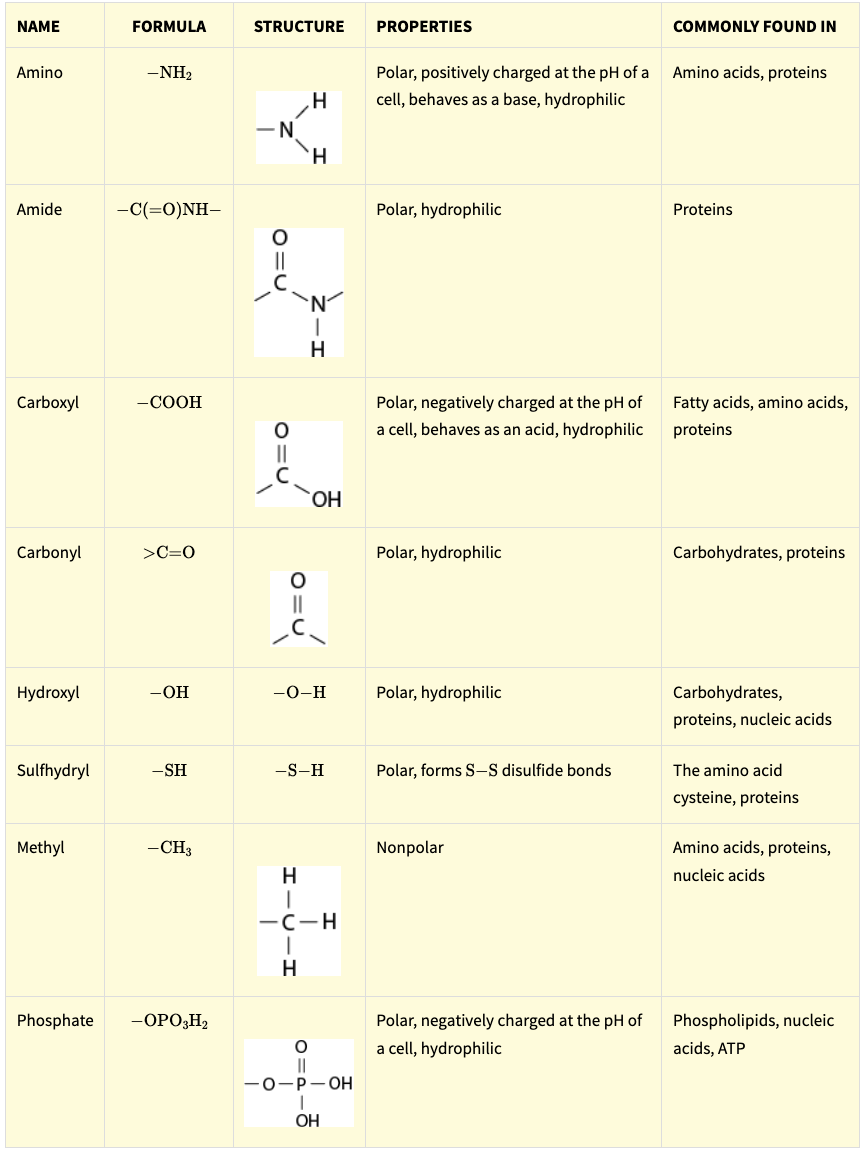
Carbon atoms can attach to functional groups that change the properties of the carbon chain
● What is the difference between a monomer and a polymer?
Monomers are simple units (e.g. amino acids or nucleotides) whereas polymers are complex molecules made of repeated monomers connected by covalent bonds
● Describe the monomers that make up proteins, nucleic acids and carbohydrates. Sketch an example of the chemical structure of each.
Proteins provide structural support and act as catalysts
Monomer: amino acid
Structure: alpha carbon, linked to amino group, carboxyl group and R group, joined to another amino acid via the C-N bond (peptide bond)
Dehydration reaction occurs as a water molecule is formed during the formation of the peptide bond
Nucleic acids encode and transmit genetic information
Monomer: nucleotide
Structure: 5-carbon sugar, a base containing nitrogen and phosphate groups, joined to another nucleotide via the phosphate group and sugar (phosphodiester bond)
Bases can either be pyrimidine (containing cytosine or thymine/uracil) or purine (guanine and adenine)
For RNA, the sugar is ribose (containing OH) whereas DNA has deoxyribose (containing only H)
Carbohydrates provide a source of energy and make up the cell wall
Monomer: sugar/monosaccharides (e.g. glucose, galactose, fructose)
Structure: unbranched carbon chains with an aldehyde/ketone group, attached via glycosidic bonds between a C-OH
Monomers form polysaccharides
Structure:
● For each of these macromolecules, what are the bonds that link these monomers to enable a polymer to form?
Covalent bonds
Proteins - peptide bonds
Nucleic acids - phosphodiester bonds
● Explain how lipids are different to the other macromolecules with regards to polymerisation? Describe how lipids are structured and sketch an example of their chemical structure.
Lipids make up cell membranes, store energy and act as signalling molecules
Not defined by a specific chemical structure but because they are hydrophobic
Form triglycerides (used for energy storage) made of three fatty acids (chain of C atoms attached to -COOH group) and glycerol
-COOH and -OH (from glycerol) attach and release water
Fatty acids can be saturated (no double bonds present) or unsaturated
Form steroids, which can form hormones such as estrogen and testosterone
Form phospholipids, which make up the cell membrane
LO2. Identify biologically important macromolecules and their functions within a cell or organism.
Additional key terms: Cellulose, complementary bases, disaccharide, double helix, fructose, galactose, glucose, glycogen, lactose, ribose, starch, sucrose, triglyceride, sugar phosphate
● List the roles of the major groups of macromolecules in organisms. How are they used within a cell/organism? Provide examples of each.
Macromolecule | General cellular functions | Examples of these functional macromolecules |
Carbohydrates |
|
|
Lipids |
|
|
Proteins |
|
|
Nucleic Acids |
|
|
● Outline the differences between unsaturated and saturated fats.
● Define a nucleotide, and what are the differences between the structure of DNA and RNA? Draw a diagram of each annotating where these differences are.
● Explain how the chemical composition and structure of DNA facilitates its biological role.
LO3. Apply theoretical knowledge of chemical bonding to the form and function of proteins.
Additional key terms: α-helix, β-pleated sheet
● Explain how the various R-groups of amino acids influence amino acids. How can this affect a protein?
● Define the primary structure, secondary structure, tertiary structure and quaternary structure of proteins. Sketch a diagram of each type.
● Describe how environmental factors such as temperature and pH may affect protein structure.
LO4. Explain how enzymes speed up and drive chemical reactions, and provide factors that can affect enzyme function.
Additional key terms: activation energy, allosteric, ATP, chemical energy, coenzyme, cofactor, energetic coupling, enzyme-substrate complex, Gibbs free energy, substrate
Gibbs free energy (DeltaG)- the amount of energy available to perform a function; the enthalpy (energy of reactants and products) minus the entropy (energy lost)
Energetic coupling - using excess energy from exergonic reactions to drive endergonic reactions
Reactions must occur simultaneously, and the net DeltaG of coupled reactions must be exergonic
Activation energy - energy required to form a transition state (intermediate molecule)
Substrate - reactants in an enzymatic reaction
Active site - highly specific region of an enzyme’s structure where the substrate binds
● Explain what is meant by potential and kinetic energy.
Potential energy - having the capacity to complete a task (energy), but storing this energy until motion occurs
Kinetic energy - energy in motion
● Define an endergonic and an exergonic reaction. Explain how they differ from each other. You may like to include an annotated diagram to help explain this.
Endergonic reaction - a reaction where the amount of energy contained in the products are higher than the energy contained in the reactants (absorption of energy, positive DeltaG)
Non-spontaneous reactions
Exergonic reaction - a reaction where the amount of energy contained in the products are lower than the energy contained in the reactants (loss of energy, negative DeltaG)
Spontaneous reaction
● Describe the effect of enzymes on chemical reactions.
Lower amount of activation energy required
Stabilises the transition state
Brings reactants together, making it more likely for the reaction to occur
● Explain how the cell can regulate the activity of enzymes, both positively and negatively.
The cell can limit access to co-factors, activators or inhibitors
Co-factors - metal ions that ensure enzymes fold correctly
Activators promote enzyme activity competitively or allosterically
Inhibitors block enzyme activity competitively or allosterically
Competitive inhibitors block the active site, preventing substrate from binding
Allosteric site - region on an enzyme that regulates its activity
Allosteric inhibitors alter the structure of the enzyme by binding to the allosteric site
● List the main factors that impact the function of enzymes and describe this impact.
Extra Notes:
DNA takes on the form of a double helix of two strands of nucleotides
Adenine and thymine form complementary pairs
Guanine and cytosine form complementary pairs
Van der Waals forces are weak bonds between slight positive or slight negative charges as electrons are constantly moving
Protein folding:
Primary structure
Sequence of a chain of amino acids using covalent bonds
Secondary structure
Folding/twisting through the formation of hydrogen bonds can lead to the existence of beta-pleated sheets or alpha helices
Tertiary structure
Quaternary structure
Enzymes lower the activation energy required
Makes the reaction more likely to proceed
Enzymes DO NOT speed up chemical reaction
Cofactors - non-protein atoms or molecules that are required fr enzyme catalytic activity
Two types:
Organic (coenzymes)
Usually vitamins (e.g. Vitamin C)
Could be non-vitamin related (e.g. ATP)
Inorganic
Usually metal ions (e.g. Mg²+)
Questions:
Are co-factors impacting the folding of the enzyme during it’s formation or is it impacting the folding of a substrate?
 Knowt
Knowt