Matter-Models and Explanation
Matter:
Anything that has mass and takes up space.
Models:
Simplified representations of complex physical phenomena.
Used to explain and predict physical behaviors.
Ancient Greek Theories
Democritus (circa 460–370 BC):
Proposed that matter is composed of small, indivisible particles called "atomos," meaning "uncuttable."
Believed that atoms were different shapes and sizes, which determined the properties of the matter they composed.
His ideas were purely philosophical and lacked experimental evidence.
Aristotle (384–322 BC):
Rejected Democritus's idea of atoms.
Suggested that matter is continuous and composed of four fundamental elements: earth, water, air, and fire.
Introduced the concept of "hylomorphism," which posits that all things are a combination of matter (hyle) and form (morphe).
John Dalton (1766–1844)
Dalton's Atomic Theory (1803):
Proposed that matter is composed of small, indivisible particles called atoms.
Each element consists of identical atoms, which differ from those of other elements.
Compounds are formed by the combination of atoms of different elements in fixed ratios.
Chemical reactions involve the rearrangement of atoms; atoms are not created or destroyed in these reactions.
Significance:
Provided the first scientific basis for the concept of the atom.
Dalton’s work laid the groundwork for modern chemistry and the law of multiple proportions.
J.J. Thomson (1856–1940)
Discovery of the Electron (1897):
Conducted experiments using cathode ray tubes.
Demonstrated that cathode rays were composed of negatively charged particles, later named electrons.
Plum Pudding Model (1904):
Proposed that the atom is a sphere of positive charge with electrons embedded within it, like raisins in a “plum pudding”.
Suggested that the positive and negative charges were distributed evenly throughout the atom.
Ernest Rutherford (1871–1937)
Gold Foil Experiment (1911):
Conducted by Hans Geiger and Ernest Marsden under Rutherford’s supervision.
Involved directing alpha particles at a thin gold foil.
Most alpha particles passed through the foil, but some were deflected at large angles.
Nuclear Model (1911):
Concluded that atoms have a small, dense, positively charged nucleus at the center.
Electrons orbit the nucleus at a relatively large distance.
Most of the atom’s volume is empty space.
Significance:
Overturned the plum pudding model.
Provided the basis for understanding atomic structure and led to the development of nuclear physics.
Niels Bohr (1885–1962)
Bohr Model (1913):
Proposed that electrons orbit the nucleus in fixed, quantized orbits or energy levels.
Electrons can jump between orbits by absorbing or emitting specific amounts of energy (quantum).
Introduced the concept of quantized angular momentum.
Significance:
Explained the spectral lines of hydrogen.
Incorporated quantum theory into atomic structure.
Laid the foundation for quantum mechanics.
Modern Atomic Model
Quantum Mechanical Model:
Developed through the work of scientists like Schrödinger, Heisenberg, and Dirac.
Describes electrons in terms of probability distributions rather than fixed orbits.
Schrödinger Equation: Describes how the quantum state of a physical system changes over time.
Heisenberg Uncertainty Principle: States that it is impossible to simultaneously know the exact position and momentum of an electron.
Orbitals: Regions in space where the probability of finding an electron is high.
Significance:
Provides a more accurate and comprehensive understanding of atomic and subatomic particles.
Essential for the development of technologies like semiconductors, lasers, and nuclear energy.
States of matter
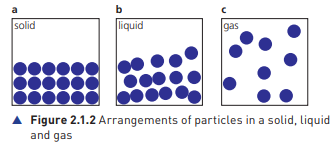
Solids
Fixed, definite shape and volume
Very strong intermolecular forces
Particles closely packed
Particles vibrate about their mean position
No fluidity
Incompressible
Particles arranged in fixed positions and in a regular pattern
Liquids
Indefinite shape and definite volume
Strong intermolecular forces
Particles are close but not packed
Particles can slide over each other
Can flow
Incompressible
Expand more than solids if heated
Gases
Indefinite shape and volume
Can flow
Particles have a large gap between them
Very weak intermolecular forces
Particles far apart from each other
Compressible
Particles are in continuous, random motion
Expand more than liquids and gases if heated
Phase Transitions
Melting: Solid to liquid.
Freezing: Liquid to solid.
Vaporization: Liquid to gas (includes boiling and evaporation).
Condensation: Gas to liquid.
Sublimation: Solid to gas without passing through the liquid phase.
Deposition: Gas to solid without becoming liquid.
Kinetic Theory of Gases
Assumptions:
Gas particles are in constant, random motion.
Collisions between gas particles are perfectly elastic.
The volume of gas particles is negligible compared to the volume of their container.
There are no intermolecular forces acting between gas particles.
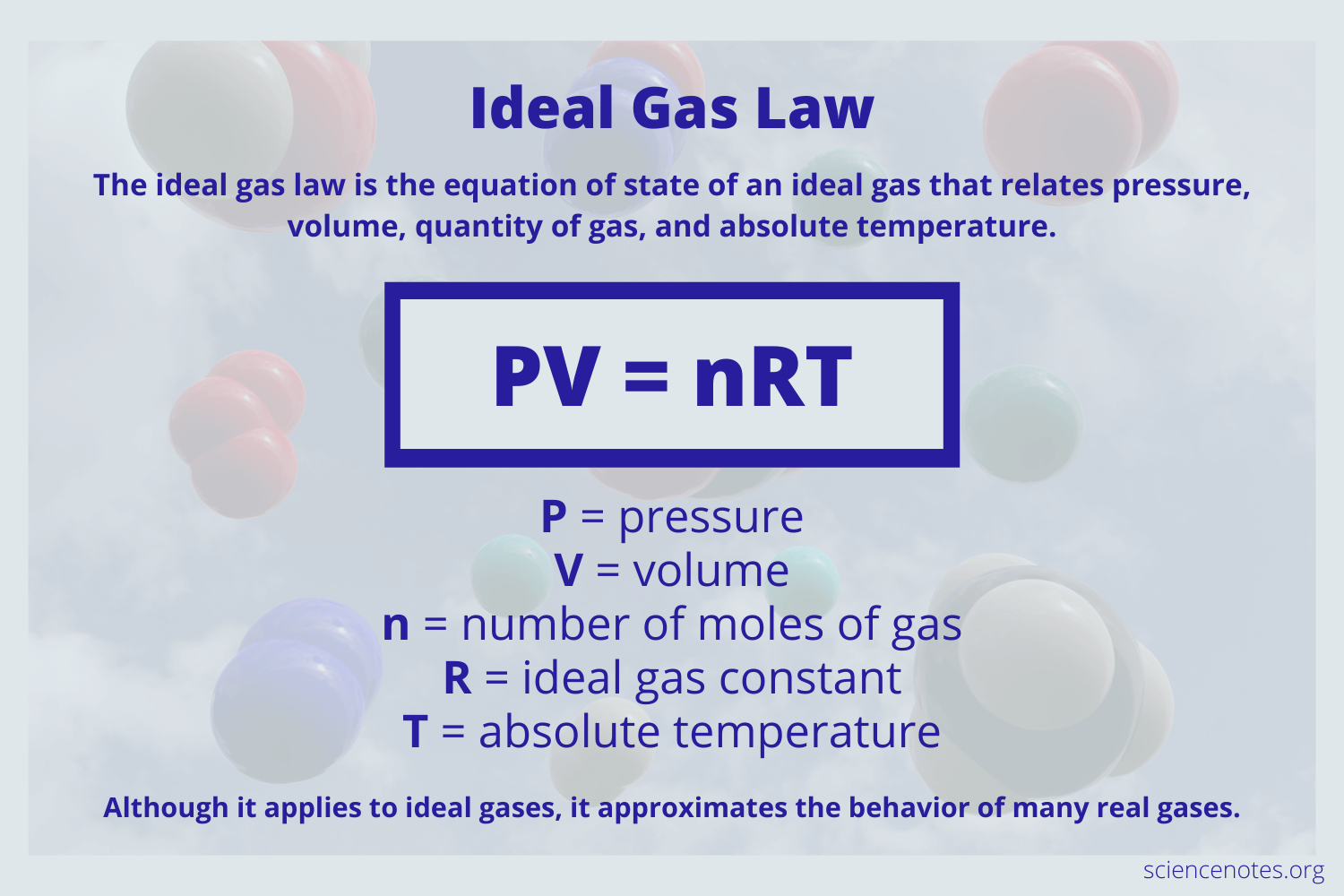
Ideal Gas Law
Thermal Properties of Matter
Temperature: Measure of the average kinetic energy of particles.
Heat: Transfer of thermal energy between substances due to a temperature difference.
Specific Heat Capacity: Amount of heat required to change the temperature of 1 kg of a substance by 1°C.
Latent Heat: Heat required for a phase change without a change in temperature.
Latent Heat of Fusion: Heat required to change a solid to a liquid.
Latent Heat of Vaporization: Heat required to change a liquid to a gas.
Density
Density is the measure of how compact the mass in an object/substance is.
Mass per unit volume
SI unit: kg/m³
ρ = m/v
ρ: density
m: mass
v: volume
Buoyancy
Buoyancy is the upward force exerted by a fluid (liquid or gas) on an object immersed in it.
This force enables objects to float or appear lighter when submerged in a fluid.
Buoyancy is a direct consequence of pressure differences within the fluid.
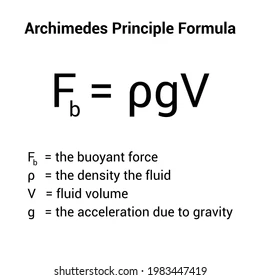
Archimedes’ Principle
Discovered by the ancient Greek mathematician and engineer Archimedes.
Archimedes’ Principle states: "Any object completely or partially submerged in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object."
Atomic and Molecular Interactions
Intermolecular Forces: Forces between molecules.
Van der Waals forces: Weak attractions between all atoms and molecules.
Dipole-Dipole interactions: Attractions between polar molecules.
Hydrogen bonding: Strong type of dipole-dipole interaction involving hydrogen and electronegative atoms like O, N, or F.
Chemical Bonds:
Ionic Bonds: Transfer of electrons from one atom to another.
Covalent Bonds: Sharing of electron pairs between atoms.
Metallic Bonds: Delocalized electrons shared among metal atoms.
Crystalline and Amorphous Solids
Crystalline Solids: Atoms arranged in a highly ordered structure, forming a crystal lattice (e.g., salts, diamonds).
Amorphous Solids: Lack a long-range order in atomic structure (e.g., glass, plastics).
Properties of Materials:
Mechanical Properties: Strength, ductility, hardness, toughness.
Thermal Properties: Conductivity, expansion.
Electrical Properties: Conductivity, resistivity.
Composite Materials: Combinations of two or more materials to enhance properties (e.g., fiberglass, concrete).
 Knowt
Knowt
