Chemistry HL S3 (3.1-3.2)
S3
3.1: Classification of Elements
(see 3.2 Periodic Trends old syllabus for more detail)
Atomic Radius
increases down a group and decreases across a period
down a group: number of occupied electron levels increase
across a period: number of occupied electron levels stay the same but the number of protons increase, increasing the nucleus’ force of attraction to the outer electrons
Ionic Radius
positive ions are larger than parent atoms due to loss of outer energy level (valence)
negative ions are smaller than parent atoms due to addition of electrons
increased electron repulsion causes electrons to move
increase in nuclear charge (number of protons) causes ionic radius to decrease
increased attraction between outer electrons and nucleus
ionic radius increases down a group due to increased amount of occupied energy levels
Ionization Energy
increases across a period and decreases down a group
Electron Affinity
decreases down a group and increases across a period
Electronegativity
decreases down a group and increases across a period
across → increase in nuclear charge increases attraction between nucleus and bond electrons
down → increases distance between nucleus and bond electrons so reduced attraction
Group 1: Alkali metals
physical properties
good conductors of electricity and heat (mobility of outer electrons)
low density
shiny grey surfaces when freshly cut with a knife
chemical properties
very reactive metals
forms ionic compounds with non-metals
forms single charged ions (X+)
reactivity increases down group (lower IE)
reaction with water: forms hydrogen and metal hydroxide
lithium: floats and reacts slowly (releases hydrogen but keeps shape)
sodium: reacts vigorously (heat produced melts the unreacted metal)
potassium: reacts more vigorously (heat produced ignites hydrogen)
Group 17: Halogens
physical properties
coloured
gradual change from gases (F2, Cl2) to liquid (Br) and solid (I2, At2)
chemical properties
very reactive non-metals
reactivity decreases down group (lower attraction)
form ionic compounds with metals and covalent compounds with non-metals
displacement reactions
the more reactive halogen displaces the less reactive halogen
halides
halogens produce insoluble salts with silver forming precipitates
Period 3 Oxides
ionic character of period 3 oxides decrease from left to right
electronegativity value approaches oxygen, so the difference is less
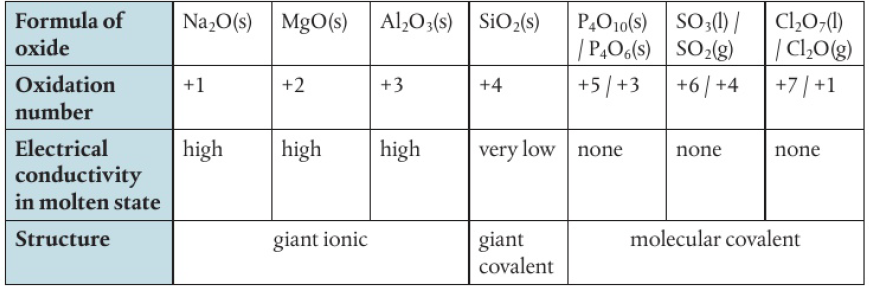
ionic oxides
dissolve in water to form alkaline solutions
reacts with acid to form a salt and water
non-metallic oxides
reacts with water to form acidic solutions
amphoteric oxides
essentially insoluble (does not affect pH when added to water)
shows both basic and acidic behaviour
Acid Rain
produced by non-metal oxides
sulfur oxides
S(s) + O2(g) → SO2(g) sulfur dioxide
H2O(l) + SO2(g) → H2SO3(aq) dissolve in rainwater
2SO2(g) + O2(g) → 2SO3(g) sulfur trioxide
H2O(l) + SO3(g) → H2SO4(aq) dissolve in rainwater (acid)
nitrogen oxides
N2(g) + O2(g) → 2NO(g) nitrogen monoxide
N2(g) + 2O2(g) → 2NO2(g) and 2NO(g) + O2(g) → 2NO2(g) nitrogen dioxide
H2O(l) + 2NO(g) → HNO2(aq) + HNO3(aq) dissolve in rainwater
2H2O(l) + 4NO2(g) + O2(g) → 4HNO3(aq) oxidized
Oxidiation States
oxidation is:
addition of oxygen
removal of hydrogen
electron loss
an increase in oxidation state
rules to assign oxidation states:
atoms in the free (uncombined) element have an oxidation state of zero
in simple ions, the oxidation state is the same as charge of the ion
oxidation states of all atoms in a neutral compound must add up to zero
oxidation states of all atoms in a polyatomic ion must add up to the charge
usual oxidation state for an element in a compound is the one most commonly found
F (fluorine) has oxidation state of -1 all the time (most electronegative)
O (oxygen) has oxidation state of +2 except in peroxides
Cl (chlorine) has oxidation state of -1 except when bonded to more electronegative ions
H (hydrogen) has oxidation state of +1 except when forming ionic hydrides
oxidation state of a transition metal in a complex ion can be found using the charge on the ligands
Transition Metals
metals in the d-block have similar physical and chemical properties
zinc is not a transition metal
has a full d sublevel in both species
physical properties
high electrical and thermal conductivity
high melting point
high tensile strength
malleable and ductile
chemical properties
forms compounds with more than one oxidation state
form a variety of complex ions
form coloured compounds
acts as catalysts when either elements or compounds
magnetic properties
only found in iron, nickel, and cobalt
due to presence of unpaired electron
every spinning electron can act as a magnet
Variable Oxidation States
transition metals display a wide range of oxidation states
all transition metals show both +2 and +3 oxidation states
maximum oxidation states increase in steps of +1 and reaches a maximum at manganese then decreases in steps of -1
oxidation states above +3 generally show covalent character
compounds with higher oxidation states tend to be oxidizing agents
Coloured Compounds
transition metal ions in solution have a high charge density
attracts water molecules which form coordination bonds with the positive ions
complex ions are formed when a central ion is surrounded by molecules/ions that possess at least one lone pair of electrons (ligands)
number of coordination bonds from the ligands to the central ion is called the coordination number
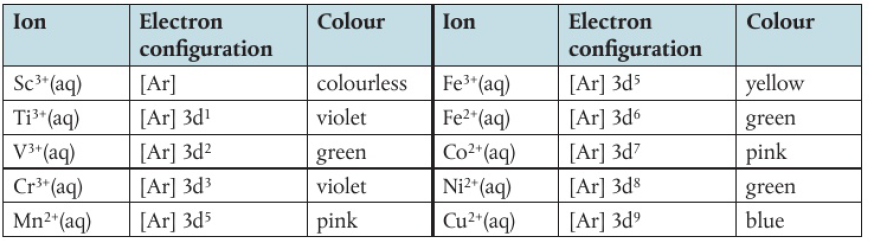
colours appear because of a spurt in the d-orbital’s energy levels
when light passes through, it excites electrons and increases the energy level for these electrons
the ions absorb some colours and reflects the ones opposite it
when the energy (light) is absorbed, the d-orbitals split into two levels
3.2 Functional Groups: Classification of Organic Compounds
empirical formula → simplest whole-number ratio of atoms
molecular formula → actual number of atoms
full structural formula → shows every bond and atom at 90°/180°
condensed structural formula → omits bonds, displays minimal information
skeletal formula → shorthand representation of a structural formula
aromatic compounds: molecules which contain a benzene ring
catenation: ability of carbon to link to itself and form chains/rings
Functional Groups
atoms/groups of atoms that are present in organic compounds and are responsible for a compound’s physical properties and chemical reactivity
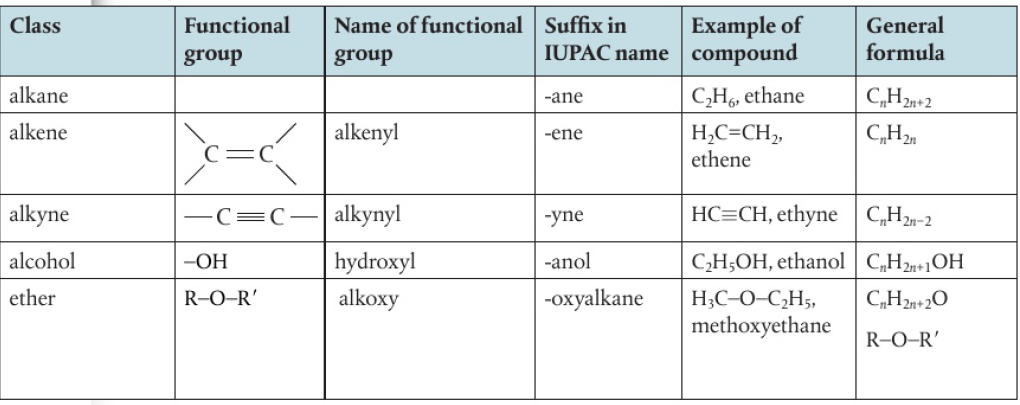
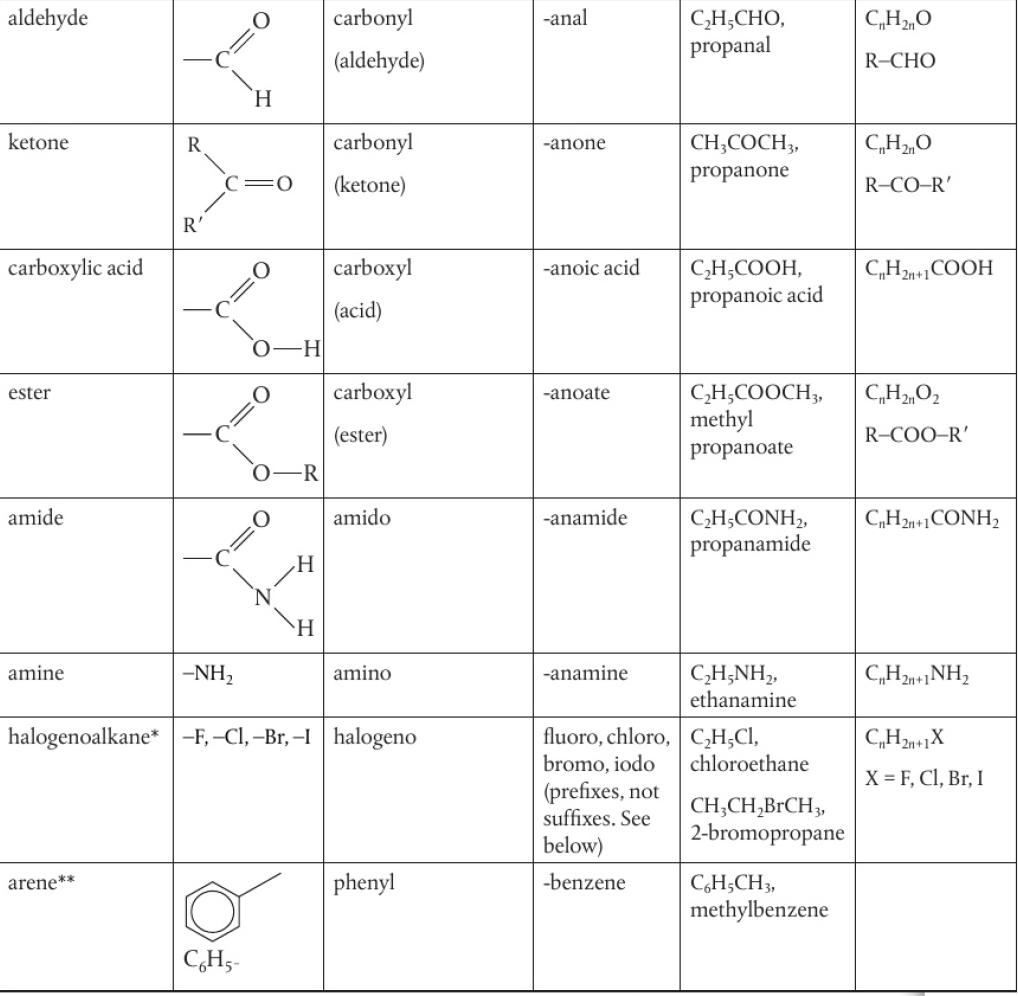
*halogen atoms are regarded as substituents as they have taken the position of a hydrogen atom
IUPAC names example: chloroethane, 2-bromopropane, etc.
**syllabus does not require knowledge of arenes as compounds but expects you to recognize the phenyl group when it is present in a structure
naming is also not required
Functional Groups: chemical reactivity
reaction pathway → several reactions to produce a target compound
product of one reaction is reactant of the next
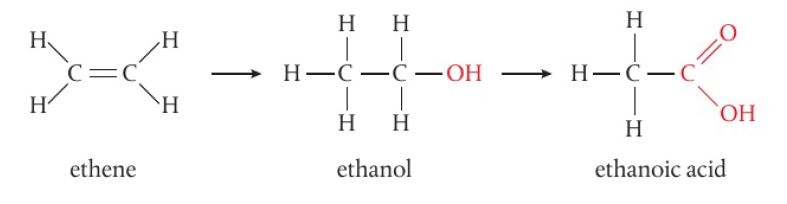
ex: ethane (CH2) → ethanoic acid (CH3COOH)
Amino acids: condensation reaction to form link
amino acids contain 2 functional groups:
amine (-NH2) and carboxylic acid (-COOH)
amino acids react together via condensation reaction
molecule of water eliminated, acid and amino groups form new bond
bond is a substituted amide link (peptide bond) forming a dipeptide
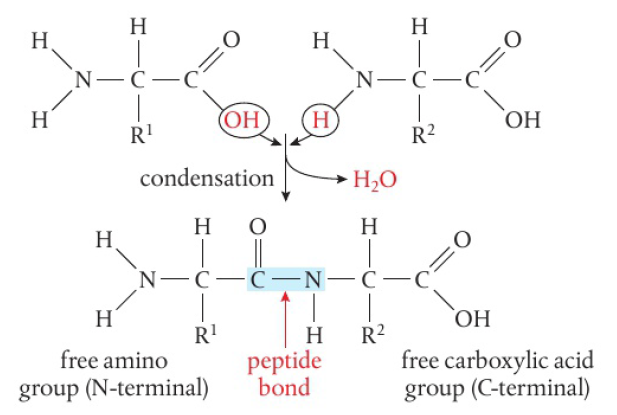
the dipeptide still has a functional group (-NH2, -COOH)
can perform a condensation reaction again, forming a tripeptide and eventually a chain of many linked amino acids (polypeptide)
Homologous Series
organic compounds are classified into ‘families’ of compounds
successive members of a homologous series always differ by CH2
ex: C2H6, C3H8, C4H10 → alkanes
members of a homologous series can be represented by the same general formula
members of a homologous series show a trend in physical properties
because they differ by CH2, carbon chains get progressively longer
so higher B.P. for example
longer chians = increased London dispersion forces
Functional groups: physical properties
most volatile → least volatile
alkane > halogenoalkane > aldehyde > ketone > alcohol > carboxylic acid
London dispersion forces → dipole-dipole interaction → hydrogen bonding
increasing strength of intermolecular attraction →
increasing boiling point →
chain length and functional groups affect intermolecular forces
polar functional groups = dipole-dopole or hydrogen bonding
IUPAC Naming
Identify the longest straight chian of carbon atoms
1=meth-, 2=eth-, 3=prop-, 4=but-, 5=pent-, 6=hex-, etc.
Identify the functional group
numbered → nuimber has to be the smallest value possible
Identify the side chains or substituent groups
halogenoalkane (-F, -Cl, -Br, -I), amine (-NH2)
Esters and Ethers
esters → form when the alkyl group of an alcohol replaces the hydrogen of a carboxylic acid in a condensation reaction
R-COOH + R’OH → R-COO-R’ + H2O
the stem comes from the parent acid but the alkyl group of the alcohol is the prefix
ex: ethanol + ethanoic acid → ethylethanoate
ethers → 2 alkyl chains linked by an oxygen atom
R-O-R’
the longer chain will be the stem and retains its alkane name
the shorter chain is regarded as a substituent and is given the prefix alkoxy
ex: methoxypropane, ethoxyethane
prefix - stem - suffix
prefix - position, number, and name of substituents
stem - number of carbon atoms in longest chain
suffix - class of compound determined by functional group
Structural Isomers
same molecular formula but different arrangements of the atoms
each isomer is a distinct compound
unique physical and chemical properties
the more branching that is present in an isomer, the lower its boiling point
reduced surface contact weakens London dispersion forces
primary carbon → attached to functional group and at least 2 hydrogen atoms
seconday carbon → attached to functional group, one hydrogen atom, and 2 alkyl groups
tertiary carbon → attached to functional group and 3 alkyl groups
Stereoisomers
atoms are attached in the same order but differing in spatial or 3D arrangements → requires 3D representation
Isomerism - structural + stereo
Stereo - configurational + conformational
Configurational - cis-trans + optical
Conformational Isomers (not needed)
spontaneously interconnect through bond rotations and so cannot be isolated seperately (usually)
some conformers of a compound may be more stable than others so are favoured → influences reactivity of the compound
Configurational isomers
permanent difference in geometry
cannot be interconverted and exist as seperate compounds with some distinct properties
Cis-trans isomers
double-bonded molecules
consists of one sigma and one pi bond (pi bond forming by sideways overlap of two p orbitals)
free rotation around this double bond is not possible
would push p orbitals out of position and pi bond breaks
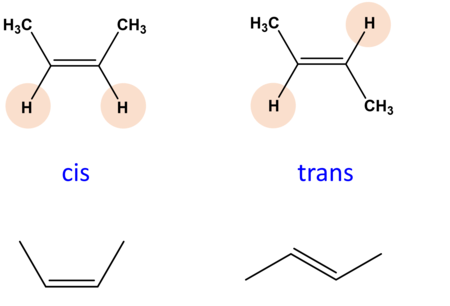
when the molecule contains two or more different groups attached to the double bond, these can be arranged to give 2 different isomers
cis → same side, trans → opposite side s
cyclic molecules
cycloalkanes contain a ring of carbon atoms that restricts rotation
bond angles are strained from the tetrahedral angles in parent alkane
Optical isomers
chiral - carbon atom attaches to 4 different atoms/groups
also known as asymmetric or stereocentre
the four groups arranged tetrahedrally with bond angles of 109.5° can be arranged in 2 different 3D configurations which are mirror images
known as enantiomers → chiral molecules
have opposite configurations at each chiral center
diastereoisomers → have opposite configurations at one or more (but not all) chiral centers
not mirror images of each other
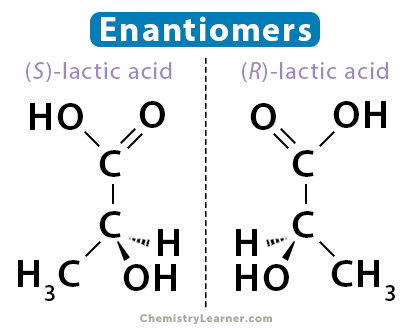
Properties of enantiomers
optical activity → interaction with light
when a beam of plane-polarized light passes through a solution of optical isomers, they rotate the plane of polarization
optically active → seperate solutions of enantiomers (at the same concentration) rotate plane-polarized light in equal amounts but opposite directions
racemic mixture → chiral compound with equal concentration of 2 optical isomers
two optical isomers’ rotations cancel out, so racemic mixtures are optically inactive
reactivity with other chiral molecules
when a racemic mixture is reacted with a single enantiomer of another chiral compound, the two components of the mixture (+ and -) react to produce different products
products have distinct chemical and physical properties so can be seperated
resolution → two enantiomers seperated from a racemic mixture
Mass Spectrometry
used to find mass of individual atoms and finding relative abundances of different isotopes → also finds relative molecular mass of a compound
Fragmentation patterns
ionization process → shooting an electron from electron gun then hitting the incident species and removing an electron
X(g) + e- → X+(g) + 2e-
X is a molecule
collision can be so energetic the molecule breaks into different fragments
fragmentation pattern is used as evidence to find the structure of a compound
peak (largest mass/charge) is molecular ion that passed without fragmenting
Infrared Spectroscopy
frequency of radiation is often measured as number of waves per centimeter (wavenumber)
radio waves can be absorbed by certain nuclei, reversing their nuclear spin (environment)
used in nuclear magnetic resonance (NMR)
microwaves cause molecules to increase their rotational energy (bond lengths)
infrared radiation is absorbed by certain bonds causing them to stretch/bend (bonds)
visible/ultraviolet light can produce electronic transitions (electronic energy levels)
x-rays are produced when electrons make transitions between inner energy levels
produce diffraction patterns (molecular/crystal structure)
Natural frequency of a chemical bond
chemical bonds are like springs/rulers
each bond vibrates and bends at a natural frequency
depends on strengths and atom masses
light atoms vibrate at higher frequencies (less weight)
multiple (stronger) bonds vibrate at higher frequencies
simple diatomic molecules can only vibrate when the bond stretches
Exciting bonds
energy needed to excite bonds occur in the infrared (IR) region
only polar diatomic molecule bonds will interact with IR radiation
presence of positive and negative charge allows the electric field component of the IR radiation to excite the vibrational energy
change in vibrational energy produces change in dipole moment
intensity of absorption depends on polarity
Stretching and Bending
in a polyatomic molecule (like water), it is more correct to consider the molecule stretching and bending as a whole, rather than considering individual bonds
ex: water can vibrate at 3 fundamental frequencies
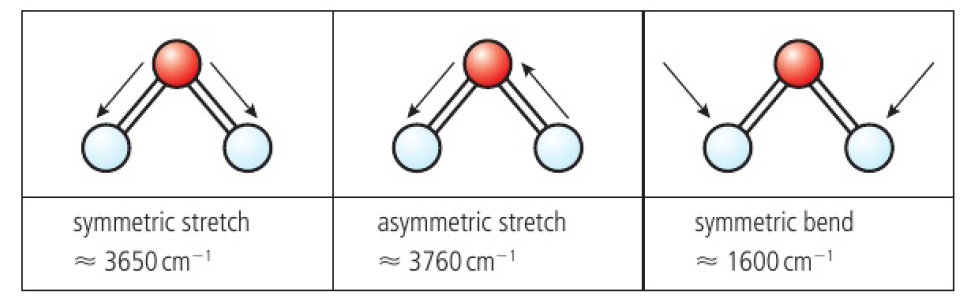
symmetric stretch, asymmetric stretch, symmetric bend
each of the modes of vibration results in a change of dipole in the molecule
can be detected with IR spectroscopy
for a symmetrical linear molecule (like carbon dioxide), there are 4 modes of vibration
symmetric stretch is IR inactive: no change in dipole moment
dipoles of both C=O bonds are equal and opposite throughout the interaction
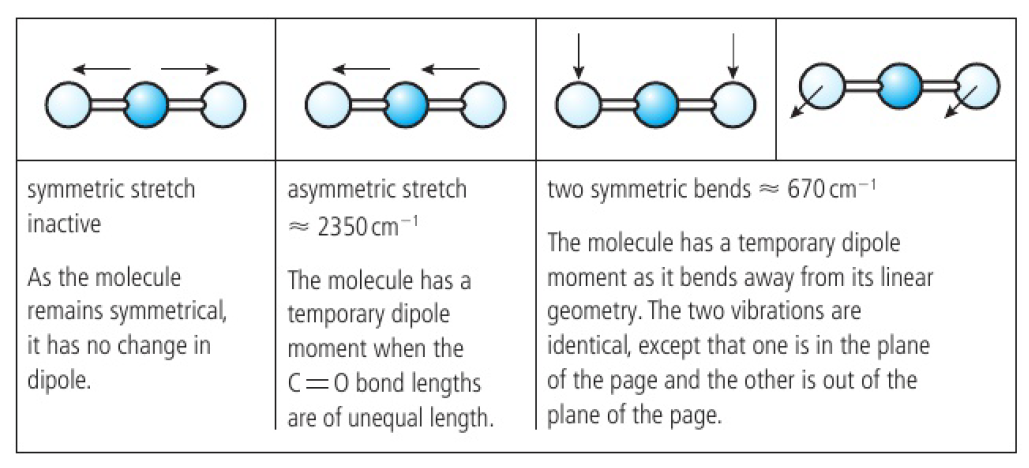
Greenhouse Gases
greenhouse effect: solar radiation passes through the atmosphere and warms the surface of the Earth. The surface radiates some of this energy as longer wavelength infrared radiation which is absorbed by greenhouse molecules which makes the air warmer, causing it to radiate heat. Some of this radiation is re-radiated back to the Earth’s surface and some back to space
the ability of a molecule to absorb infrared radiation depends on the change in dipole moment that occurs as it vibrates
some greenhouse gases are much more effective than others in absorbing IR radiation
global warming potential: amount of infrared radiation that one tonne of a gas would absorb compared to the amount that would be absorbed by one tonne of carbon dioxide
depends on effectiveness and atmospheric lifetime of the gas
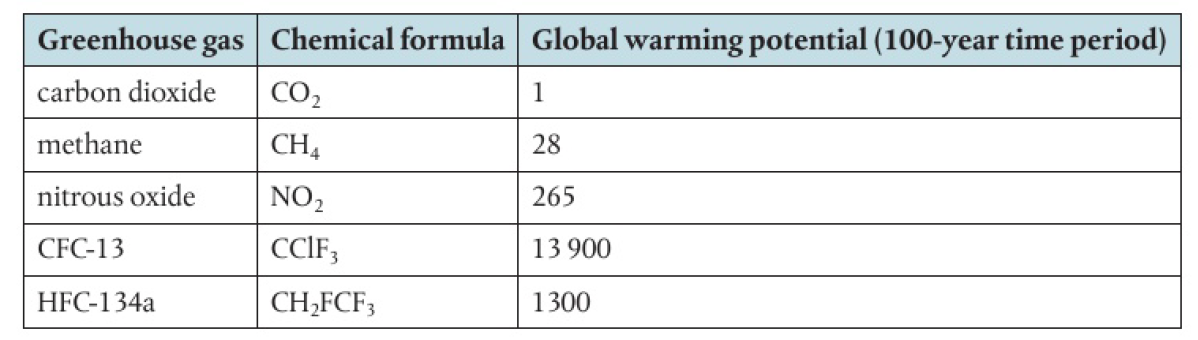
Wavenumbers
absorption of certain wavenumbers of IR radiation helps to identify bonds in a molecule
some bonds can be identified by shapes of their signal
ex: O-H bond is broad, C=O is sharp
hydrogen bonding broadens IR absorption so can be detected
ex: O-H in carboxylic acids have broader absorption
molecules with several bonds can vibrate in many different ways and with different frequencies
complex pattern can be used as a ‘fingerprint’ to be matched
comparison of spectrum of a sample with a pure compound can be used as a test of purity
Nuclear Magnetic Resonance Spectroscopy (NMR)
nuclei of atoms with an odd number of nucleons (H, C, F) have a property called nuclear spin and behave like tiny bar magnets
when placed in an external magnetic field, these nuclei can exist in two distinct energy levels
depending on whether magnetic field is aligned with/opposed to the external magnetic field
energy gap between the energy levels is very small and only requires absorption of low-energy radio waves to close the gap between energy levels
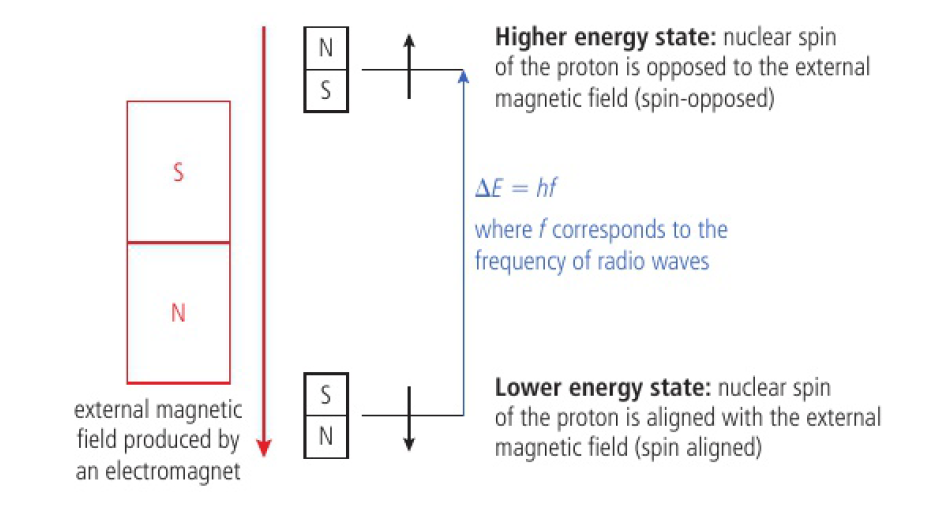
as electrons shield nucleus from full effects of external magnetic field, differences in electron distribution produce different energy seperations between the two spin energy levels
nuclei in different chemical environments produce different signals
proton = hydroegn because hydrogen has 1 proton
Magnetic Resonance Imaging (MRI)
application of NMR spectroscopy
uses H’s magnetic moment
with a powerful magnet, radio waves are used to generate an electronic signal that can be decoded to produce images
useful in diagnosis of living tissue due to hydrogen in water
H NMR Spectroscopy
NMR provides:
number of signals in the spectrum
position/chemical shifts of each signal
size/integrated area of each signal
splitting pattern observed for each signal
gives information on chemical environments and therefore structure
Chemical environments
hydrogen nuclei (protons) that have the same chemical environment are said to be equivalent as they give the same signal in NMR
number of singals observed therefore depends on number of chemical environments
Chemical shifts
position where a signal appears in NMR spectrum is measured in terms of chemical shift which has units of parts per million (ppm)
the closer a hydrogen atom is to an electronegative atom, the more pronounced the electron-withdrawing effect and the higher chemical shift observed
the high electronegativity effectively pulls electrons away from the hydrogen atoms thus deshielding the hydrogens’ nuclei
nuclei are now more susceptible to effects due to external magnetic field
hydrogen nuclei in particular environments have characteristic chemical shifts
found in section 21 of data booklet
Splitting patterns
individual signals in NMR do not consist of a single peak
signals are split/resolved into distinctive patterns
splitting occurs as the effective magnetic field experienced by particular nuclei is modified by the magnetic field produced by neighbouring protons
spin-spin coupling
the number and intensity of lines produced are easily predicted
based on the number of neighbouring hydrogens involved in coupling
number of lines: n+1 → n=number of hydrogen atoms on the neighbouring carbons
intensity: pascal’s triangle
pattern will continue for each additional proton on neighbouring carbons
protons bonded to the same atom do not interact as they are equivalent and behave as a group
protons on carbon atoms not adjacent to each other do not generally interact as they are too far apart for their magnetic fields to interact
alcohol protons (OH) typically do nto engage in spin-spin coupling
signals for OH protons are not split and appear as singlets
OH protons are not counted when applying the n+1 rule
 Knowt
Knowt