3.2 Properties of Solids
Important Vocabulary/Terms
Heat of fusion
Heat of Vaporization
Vapor Pressure
Covalent Network Solids
Biomolecules and Synthetic Polymers
Metals
Properties of Ionic Solids
Solubility and Conductivity:
Most are soluble in polar solvents.
They conduct electricity when molten or dissolved in a polar solvent, as the charged particles are free to move.
The higher the concentration of ions in a solution, the higher the electrical conductivity.
Strong bonds:
Very strong Coulombic forces of attraction.
High melting points
Very hard
Low volatility
Cleave Along Planes:
Brittle 3D structure
Ions line up in a repetitive pattern that maximizes attractive forces and minimizes repulsive forces.
Not malleable or ductile.
Properties of Molecular Solids
Most molecular solids do not conduct electricity when molten or dissolved in water.
The individual molecules have no net charge, as their valence electrons are tightly held within covalent bonds and lone pairs.
Acid molecules that can ionize and conduct electricity.
Most molecular solids are held together by intermolecular forces, which are much weaker than ionic or covalent bonds.
They have much bigger higher vapor pressures than ionic solids.
They have much lower melting and boiling points than ionic bonds.
Molecular Solids
Molecular shape plays a role in their physical state.
In a solid, molecular are held together in a regular pattern by intermolecular forces that attempt to maximize attractions and minimize repulsions.
Change of State: Heating Curve
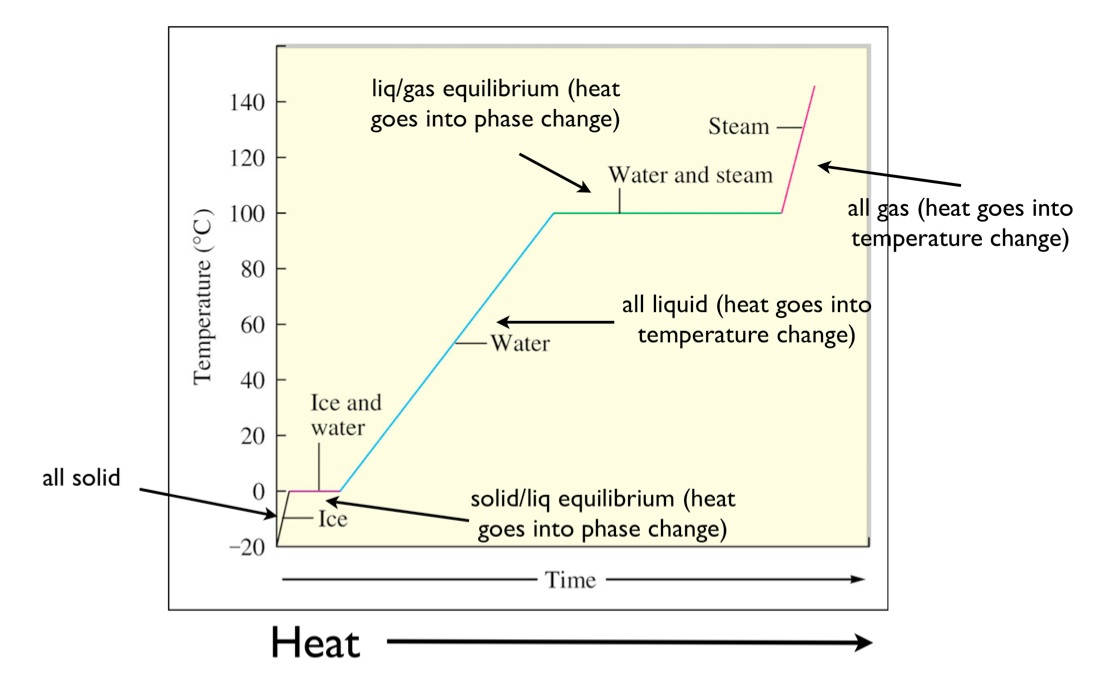
Heat of Fusion (^H fus)
^H fus - The heat absorbed 1 mole of a solid liquefies.
Effect Energy is required to expand/seber
his is why molar heat of fusion, ^H fus, values are always positive.
molting is always an endothermic process.
^H fus For Ionic Compounds
as ionic bonds are much stronger than intermolecular forces, the ^Hfus values for ionic compounds are very large.
The melting and boiling temperatures for ionic compounds are very high.
Molecular Liquids
In a Liquid, intermolecular forces also attempt to maximize attractions and minimize repulsions. These forces are strong, but not as strong as they are in a solid, so the molecules have more freedom to move.
Heat of Vaporization (^Hvap)
^Hvap - the heat absorbed as 1 mole of a liquid becomes gaseous.
Energy is required to sever the intermolecular forces of attraction, as a molecule move from the liquid to the gas phase.
Vaporization is always endothermic, so ^Hvap values are positive.
Ideally, there are no intermolecular forces of attraction between as particles.
Vapor Pressure
When molecules leave the surface of a liquid to enter the gas phase, they exert the pressure.
The vapor pressure exerted depends on the rate of evaporation per unit area of the liquid’s surface.
Rate of evaporation and vapor pressure increases as temperature increases.
When two substances are at the same temperature, the rate of evaporation and vapor pressure will be higher in the substance that has weaker intermolecular forces.
Boiling Points
A liquid boils when its vapor pressure equals atmospheric pressure.
Evaporation occurs inside the liquid when the vapor pressure equals the atmospheric pressure.
Bubbles are water vapor, not ‘air’.
Boiling Points of Water
Boiling points decrease as elevation increases
Boiling Points of Different Liquids
The vapor pressure on the surface of a liquid depends on the strength of its intermolecular forces.
A molecule restrained by strong intermolecular forces requires more energy to break free from the liquid state.
When a system requires more energy to cause its molecules to enter the gas phase, it will also require more energy to cause its vapor pressure to equal the atmospheric pressure.
Sublimation
Solids can evaporate and have a vapor pressure
As intermolecular forces are stronger in solids, the vapor pressures of solids are normally low.
Solids with high vapor pressures, have relatively weak intermolecular forces.
Vapor Pressures of Ionic Solids
Ionic compounds have very low vapor pressures and very high boiling points.
Strong Coulombic interactions between cations and anions.
Covalent Network Solids
Always composed of one two nonmetals held together by networks of covalent bonds.
Carbon group elements often form covalent network solids, as they can form four covalent bonds.
Very high melting points and normally very hard, as atoms are covalently bonded with fixed bond angles.
e.g. A diamond is one molecule
Many carbon atoms bound together with sp³ hybrid orbitals.
Each carbon makes a single covalent bond with 4 other carbon atoms.
Very hard and very high melting point (3550 C)
Graphite
Each carbon forms three sp² hybrid orbitals that bond with three other carbon atoms.
These sheets sit on top of one another.
Delocalized pi bonds between sheets.
Weak pi bonds and london dispersion forces allow sheets to slide over one another (pencils)
If hooked up to a potential difference, electrons will flow.
High melting point, as covalent bonds between carbon in each layer are relatively strong.
SiO_2 (The empirical formula for quartz)
Network of SiO_4 tetrahedra
Every silicon atom is covalently bonded to four oxygen atoms.
Every oxygen atom is covalently bonded to two silicon atoms.
Si SiC
A 3-D network with a geometry that is similar to that of a diamond.
SiC (The empirical formula for Quartz)
A covalent network of SiO_4 tetrahedra.
Every silicon atom is covalently bonded to four oxygen atoms.
Every oxygen atom is covalently bonded to two silicon atoms.
Si (Forms a covalent network with itself)
A 3-D network with a geometry that is similar to that of a diamond.
Biomolecules - Protein Structures
H-Bonds form between oxygens and the hydrogens that are bonded to nitrogens, within the same chain.
The following secondary structures can form:
The a-helix structure is held together by H-Bonds.
The B-pleated sheet structure is also held together by H-bonds.
Tertiary structures are caused by intermolecular interactions between R groups.
Quaternary structures are caused by intermolecular interactions between different chains.
Protein Function
The overall surface shape determines function.
A groove creates the opportunity for a protein to interact with other molecules.
Enzymes break down other molecules through this type of interaction.
Vocab:
Active Site - Groove
Substrate - Molecule to be broken down by enzyme
Enzyme - Substrate complex
Water soluable proteins have polar ‘R’ groups that face out and non-polar ‘R’ groups that face in.
H-bonds form with water.
Synthetic Polymers - Polyethylene
Plastics are non-polar, so they are held together by london dispersion forces.
London dispersion forces hold chains together.
Properties of Synthetic Polymers
Plastics are generally flexible solids or viscous liquids.
Heating plastics increase flexibility and allows them to be molded.
Molecular vibrations increase
London Dispersion forces weaken
properties of synthetic polymers can be modified by manipulating their structures.
Metallic Solids
Bonding is not covalent
Not enough electrons to fill octets
Bonding results from attractions between nuclei and delocalized valence electrons moving throughout the structure.
Bond strength increases as the number of bonding electrons increases.
Metallic Bonding
Electron Sea Model
Nuclei and inner core electrons are localized, while valence electrons are free to move around the solid.
Conducts electricity
Conducts heat
Malleable and Ductile
Lacks directional bonds
Steel - An Interstitial Alloy
Carbon fills some spaces between iron atoms.
Interstitial carbon atoms make the lattice more rigid, less malleable, and less ductile.
Retains a ‘sea of electrons’ so it can conduct electricity.
Brass - A Substitutional Alloy
zinc atoms are substitued for some copper atoms.
Substitutional alloys remain malleable and ductile.
Retains a ‘sea of electrons’ so it can conduct electricity.
 Knowt
Knowt