CEN 304 EXAM 1
Match the following terms and their definitions
when there is only one phase present and the substance cannot be removed from the liquid without accomplishing a phase change it is said to be:
Dissolved
These are large enough to settle out of solution or be removed by filtration
Suspended solids
Removed by centrifugation or filtration through very small pore spaces- not settling or normal filtration; Measured by using turbidity Nephelometric Turbidity Units (NTU)
Colloidal particles
True or False: 1ppmm equals 1 ug of pollutant per kg of solid (mg/kg)
False
Al3+ has how many equivalents per mole?
3
Alkalinity is typically expressed as what?
CaCO3
Henry’s Law constant refers to chemical equilibrium between air and what?
Water
True or False: acidic solutions have pH<7
True
Match the chemcial reaction type with the listed examples:
precipiration-dissolution: Hard water forms scale on indoor plumbing
Acid-Base chemisty: Disinfection using hypochlorous acid
oxidation-reduction: Iron corrosion
volatilization: Fumes from gasoline
air-water equilibrium: A gas dissolving in a liquid
Alkalinity is a water’s capacity to neutralize acids, while buffering capacity is the ability of water to resist changes in pH when an acidic or alkaline material is added.
In most natural waters with pH= 6-8, the alkalinity is mostly due to what chemical species?
HCO3-
What portion of the figure that would be the control volume for this set up?
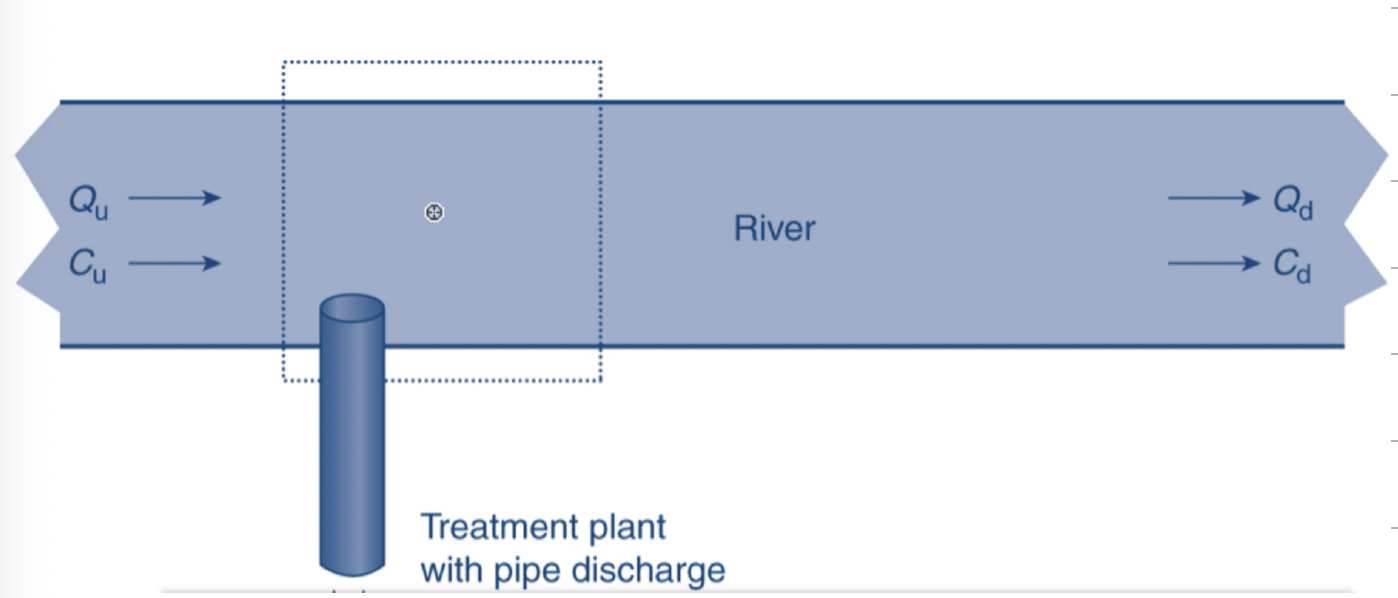
What portion of the figure that would be the control volume for this set up?
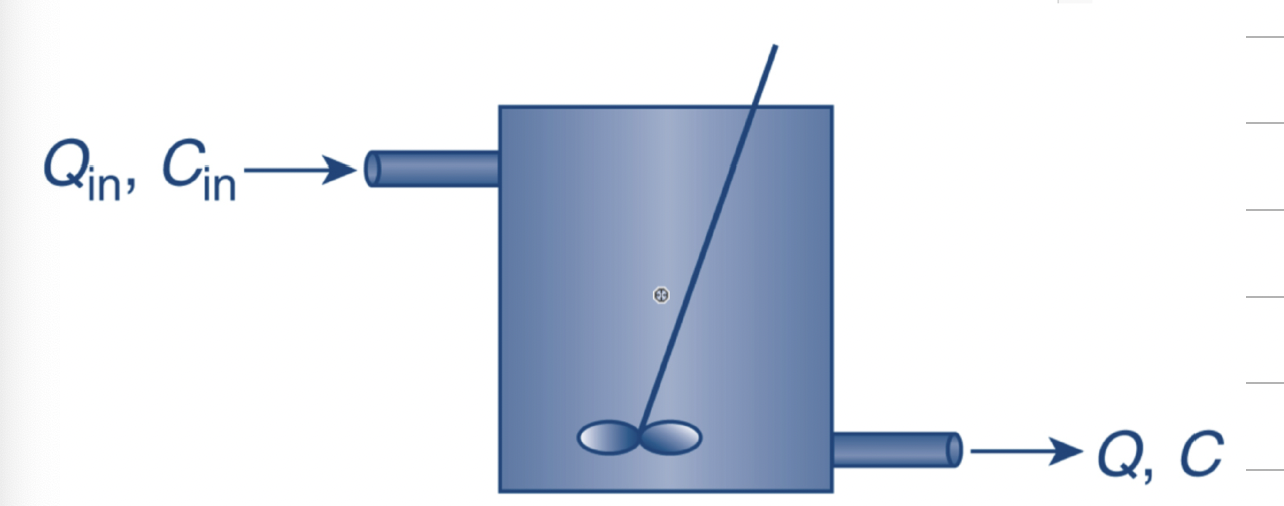
What are the terms in a mass balance equation?
Q*C
m (m dot)
dm/dt
Q* Density
True or False: The following equation represents 1st order decay: dC/dt= -k
False
State the following rate constant units indicate that a reaction is first order.
/min
/s
The graph below represents a 1st order reaction
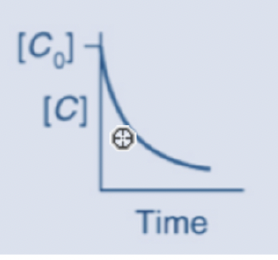
The following equations would be used to calculate the half life of a chemical that degrades according to a 1st order reaction
0.693/k
You cannot treat a non-steady state problem as a steady state problem, but you can treat a steady state problem as a non-steady state problem
True or False: For a steady-state problem, the concentration is constant within the control volume.
True
A sewer has been draining into a river for a long period of time. Would this be considered steady or non-steady state?
Steady state
A sewer just started draining into a river. Would this be considered steady or non-steady state?
non-steady state
True or False: For a PFR, there is a mixing in the axial direction
False
Since there is a high inlet concentration that is diluted immediately for CMFRs, the reaction rate is low compared to a PFR
A PFR model is most appropriately used to model the following:
pipe
A CMFR model us most appropriately used to model the following:
Lake
irrigation channel
river
True or False: A PFR can achieve a higher removal efficiency for a given volume compared to a CMFR
True
Greenhouse gases absorb most strongly in which part of the electromagnetic spectrum?
infrared
True or False: Greenhouse gases occur naturally
True
What is the name of the federal legislation first passed 1970 that defined the federal government’s responsibilities for protecting and improving the nation’s air quality?
Clean Air Act
What are the Criteria Air Pollutants?
CO
NO2
PM10
O3
SO2
Pb
Define the following terms:
Energy: Capacity to do useful work
Work: Force acting on a body through a distance
Power: Rate of doing work
Specific heat: Quantity of het required to increase unit mass of a substance by one degree
Latent heat of fusion: Energy to cause phase change from solid to liquid
Latent heat of vaporization: Energy to cause phase change from liquid to gas
CO, HC, NOx represents the inputs for a catalytic converter that are removed while CO2, H2O, N2 represents its outputs
True or False: The EPA regulated based on the AQI
False
True or FalseL NAAQS are enforced by the EPA
False
The goal of the Cap and Trade system for acid rain was to reduce what following chemicals?
SOx
NOx
For adsorption systems, a collected pollutant remains in the adsorption bed, until bed becomes saturated and pollutant leaks out. This is called what?
Breakthrough
Absorption which occurs in scrubbers is governed by partial pressure of the pollutant and Henry’s Law, while Adsorption can be done with active carbon is governed by the Langmuir isotherm.
Renewable resources can be replenished by natural processes with a human timescale while sustainable resources can be used for a very long time without causing substantial harm to humans or the natural environment
True or False: The price of solar cells is increasing
False
What renewable resources are considered intermittent?
Solar
Wind Power
True or False: As one rises up into the atmosphere, the temperature always decreases.
False
During unstable conditions vertical movement of an air parcel in the atmosphere is encouraged upward or downward, while during stable conditions vertical movement of an air parcel is discouraged
The following model shows the atmospheric temperature profile on the left that is most likely to lead to the looping plum seen on the right
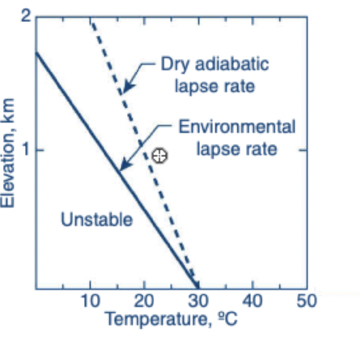
True or False: A gamma value of -5 C/km represents a stable atmosphere.
True
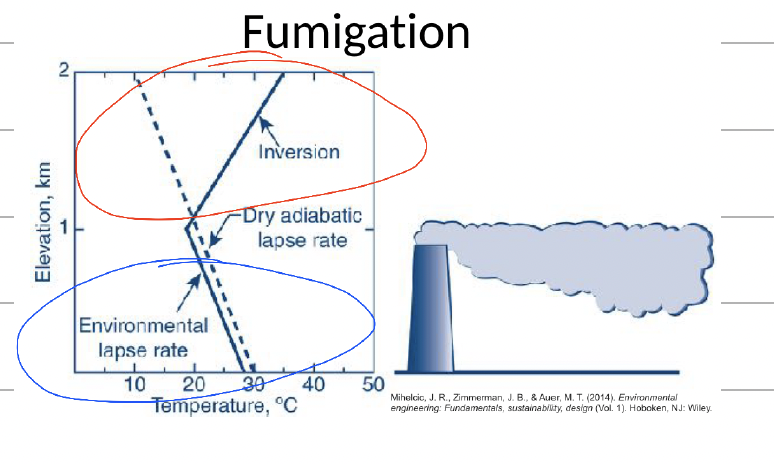
The following model should the atmospheric temperature profile on the left that is most likely to lead to fumigation as seen on the right.
Pro and Cons of Hydroelectric Power
Pros:
Highly efficient in terms of emissions
long operating life with proper maintenance
flood control
Cons
Energy output depends on the amount of precipitation
high initial costs
needs regular maintence
Pros and Cons of Solar
Pros
Infinite Supply/Renewable energy source
Lower electricity bills
reduce carbon footprint
Cons
Expensive startup costs
sun is not always there
need to mine for batteries
Pros and Cons of Biomass(firewood)
Pros
Renewable due to the organic materials
planting tree/growing forests could offset the CO2
Using local firewood diminishes transportation cost
Cons
Harmful pollutants are released
potential concern about deforestation
expensive
Pros and Cons of Biofuels (corn-based)
Pros
lower greenhouse gas emmissons
waste reduction
reducted air pollutants
Cons
high production cost
less efficient than fossil fuels
land use/deforestation
Pros and Cons of Geothermal
Pros
Small land footprint
renewable and sustainable
pollution decrease
Cons
high initial costs
location dependence
scaling limitations