
Enzyme Inhibitors
3 Types of Enzyme Inhibitors
Competitive
Non-competitive
End-product
Competitive Inhibitors
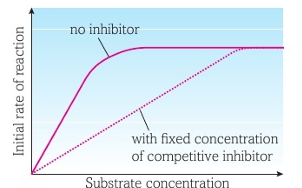
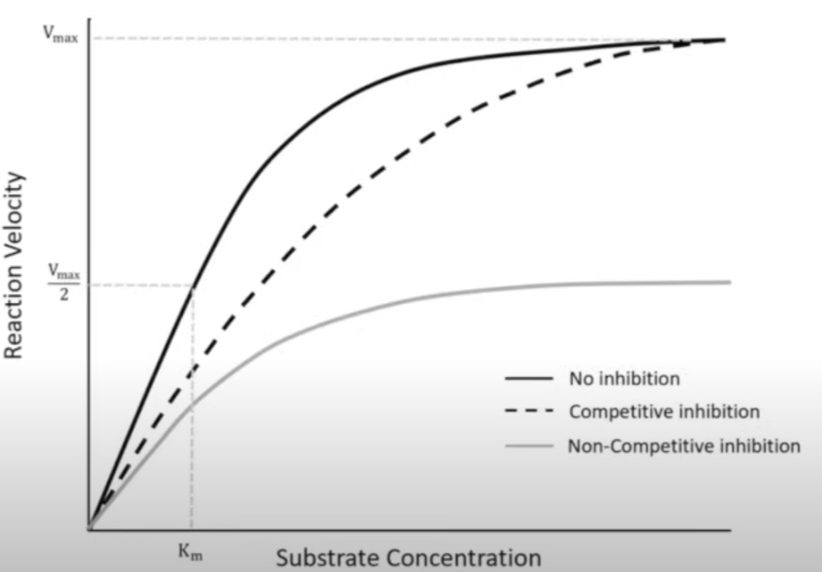
Competitive inhibitors have the same or very similar shape to substrate thus are also complementary to the active site, allowing them to actually bind to the active site.
This prevents formation of ESC’s and rather leads to formation of Enzyme-Inhibitor complexes (EIC’s), reducing rate of reaction.
2 types:
Reversible- can be removed from enzyme.
Irreversible - cannot be removed from enzyme.
Most competitive inhibitors are reversible, and in actuality, if substrate conc. is high enough, substrates can knock inhibitor molecules out of the active site, and form ESC’s thus increasing rate of reaction.
If an irreversible inhibitor binds to an enzymes active site, it becomes an inactivator.
Non-Competitive Inhibitors
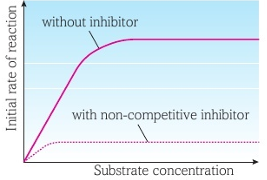
Bind to allosteric site which disrupt enzymes 3o thus changing the shape of the active site, making it no longer complementary to the specific substrate, meaning that ESC’s cannot form at all, regardless of how much substrate is added.
This means that the max. rate of reaction is reduced significantly, fewer ESC’s form leading to a lower rate of reaction, and even if high substrate conc. is present, the rate of reaction wont return to its normal uninhibited max.
The more non-competitive inhibitors present, the greater the degree of inhibition, as more active sites become distorted, thus unable to form ESC’s.
Can also be reversible or irreversible.
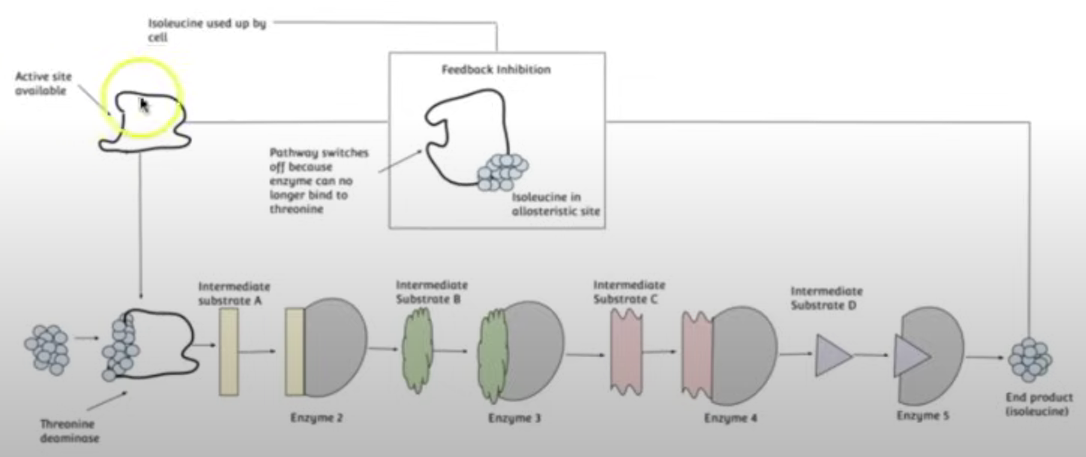
End-Product Inhibition
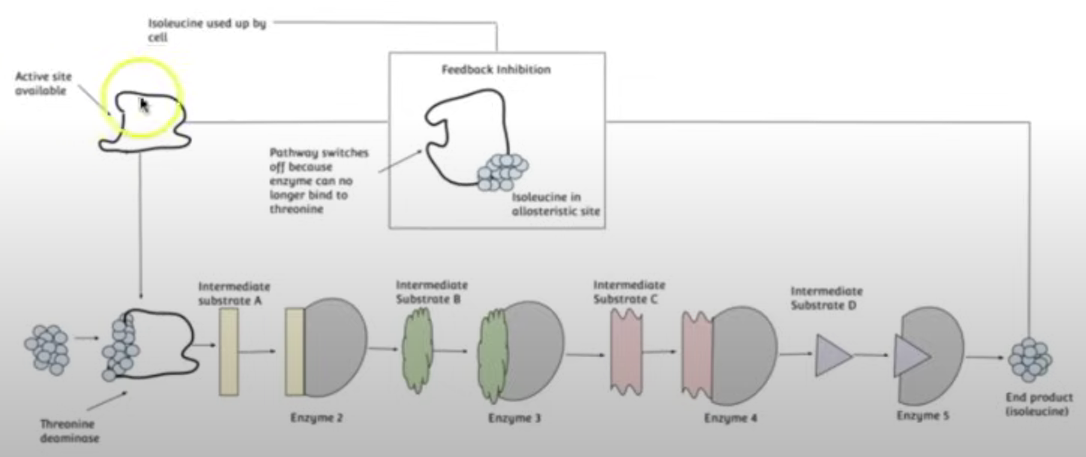
Prevents wastage of resources by regulating its own reaction, an example of negative feedback.
This happens due to the end product of the enzyme-catalysed reactions being a reversible inhibitor for the other enzymes involved in the reaction.
The end product of the series of enzyme-catalysed reactions binds to the first enzyme at its allosteric site, which is non-competitive inhibition, but reversible, leading to the first enzyme’s active site to be altered meaning it doesn’t form ESC’s w/ the substrate and the series of reactions no longer continues.
However, when the end product becomes scarce it unbinds from the first enzyme’s allosteric site allowing it to form ESC’s w/ the substrate allowing the series of enzyme-catalysed reactions to continue leading to the production of more end product molecules.
Enzyme Inhibitors
3 Types of Enzyme Inhibitors
Competitive
Non-competitive
End-product
Competitive Inhibitors
Competitive inhibitors have the same or very similar shape to substrate thus are also complementary to the active site, allowing them to actually bind to the active site.
This prevents formation of ESC’s and rather leads to formation of Enzyme-Inhibitor complexes (EIC’s), reducing rate of reaction.
2 types:
Reversible- can be removed from enzyme.
Irreversible - cannot be removed from enzyme.
Most competitive inhibitors are reversible, and in actuality, if substrate conc. is high enough, substrates can knock inhibitor molecules out of the active site, and form ESC’s thus increasing rate of reaction.
If an irreversible inhibitor binds to an enzymes active site, it becomes an inactivator.

Non-Competitive Inhibitors
Bind to allosteric site which disrupt enzymes 3o thus changing the shape of the active site, making it no longer complementary to the specific substrate, meaning that ESC’s cannot form at all, regardless of how much substrate is added.
This means that the max. rate of reaction is reduced significantly, fewer ESC’s form leading to a lower rate of reaction, and even if high substrate conc. is present, the rate of reaction wont return to its normal uninhibited max.
The more non-competitive inhibitors present, the greater the degree of inhibition, as more active sites become distorted, thus unable to form ESC’s.
Can also be reversible or irreversible.


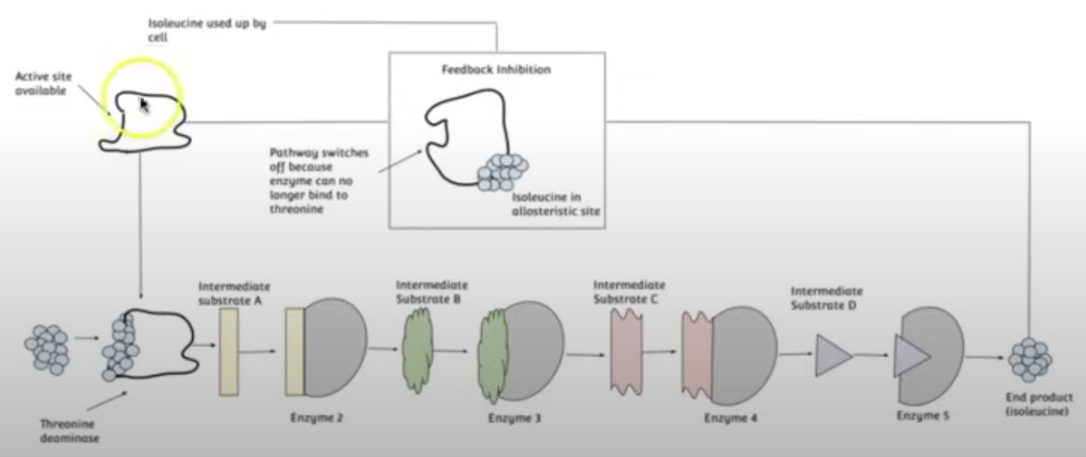
End-Product Inhibition
Prevents wastage of resources by regulating its own reaction, an example of negative feedback.
This happens due to the end product of the enzyme-catalysed reactions being a reversible inhibitor for the other enzymes involved in the reaction.
The end product of the series of enzyme-catalysed reactions binds to the first enzyme at its allosteric site, which is non-competitive inhibition, but reversible, leading to the first enzyme’s active site to be altered meaning it doesn’t form ESC’s w/ the substrate and the series of reactions no longer continues.
However, when the end product becomes scarce it unbinds from the first enzyme’s allosteric site allowing it to form ESC’s w/ the substrate allowing the series of enzyme-catalysed reactions to continue leading to the production of more end product molecules.



 Knowt
Knowt