Toxicity
Toxicity is the degree to which a chemical substance/mixture of substances can damage an organism or cell. Depends on both intrinsic and extrinsic factors. Intrinsic factors include genetic polymorphisms, sex, immune system and circadian rhythm. Extrinsic factors include dose, exposure route, duration and co-exposure to other chemicals.
Types of toxicity include chemical, biological, physical, radiation and behavioural toxicity. Chemical toxicity measures harmful effects of a substance to cause harmful health effects eg chlorine gas and cyanide. Biological toxicity measures harmful effects of biological agents eg toxins and venoms.
Mutagen = anything that causes a change in cell DNA
Carcinogenic = causes mutation that causes mutation which promotes the formation of cancer
Teratogenic = causes structural/functional change by altering foetal tissue development
Reactive oxygen species (ROS) underpin toxicity mechanism of many compounds, and are generated by the reduction of oxygen.
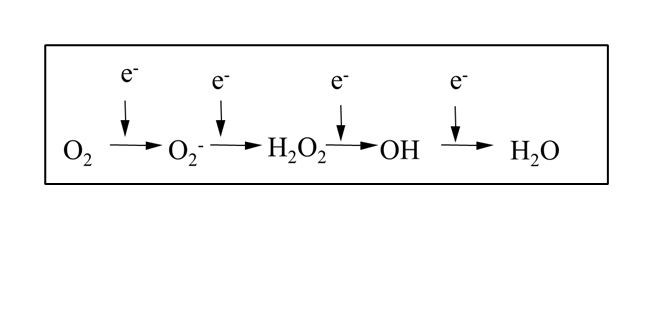
ROS can be endogenous or exogenous. Endogenous ROS are byproducts of normal cellular activity eg oxidative phosphorylation, transition metal ions and thymidine/polyamine catabolism. Exogenous ROS are byproducts of xenobiotic metatolism or radiation therapy eg ionisation of water following radiation therapy, xenobiotics compromising ROS antioxidant defense systems or metabolised to a free radical.
ROS can be radicals or non-radicals. A free radical is an atom, molecule or ion with at least one unpaired valence electron eg hydroxyl radicals (most reactive) or superoxide anions. Non radical ROS includes hydrogen peroxide or hypochlorous acid (HOCl).
ROS cause toxicity by direct damage to macromolecules by reacting with them eg to DNA by oxidising bases, lipids by peroxidation, or carbonylation of proteins.
Cells reduce ROS with antioxidants. Enzymatic antioxidants include superoxide dismutase, catalase, thioredoxin, redox proteins or peroxidoxin. Nonenzymatic include vitamin C, flavonoid, carotenoid, glutathione and vitamin E.
Glutathione, or GSH, is a tripeptide of glutamate cysteine and glycine. There’s an unusual gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. Very high expression of glutathione in cells, and in healthy cells 90% is in the reduced form.
Glutathione directly quenches some free radicals by reacting with the thiol group. Is also cofactor for many antioxidant enzymes and regenerates vitamin C and E.
Metabolism of xenobiotics occurs to recycle useful structures and to make them more soluble to aid excretion. Mainly carried out in the liver in the hepatic portal vein. Many of the enzymes used in metabolism evolved to deal with compounds in the diet. As a result of metabolism the compound is either activated (more toxic) or detoxified (less toxic).
H2O2 + 2GSH → GSSG + 2H2
Phase I metabolism = modification (oxidation/reduction/hydrolysis/hydration)
Phase 2 metabolism = conjugation (to glutathione/sulphate/glucoronide or acetylation)
Phase III metabolism = excretion
Phase I enzyme examples:
oxidation eg cytochrome P450 monooxygenases (CYPs)
reduction eg quinone reductase (NQO1)
hydrolysis eg esterases/amidases
hydration = epoxide hydrolase.
These enzymes introduce a polar group into a substrate. They aid solubility but can increase reactivity - prodrug activation often occurs in phase I.
Example phase II enzyme is glutathione S-transferases. They conjugate the molecule to a charged species. The product has a higher molecular weight and tends to be less active. The polar/charged product can’t diffuse across the cell membrane so requires active transporters.
Enzymes for phase III metabolism are xenobiotic transporters, multi drug resistance protein 1/2, transmembrane proteins and ATP dependent efflux pumps. These proteins have a physiological role in lipid translocation and are upregulated in many cancers.
Benzo[a]pyrene is a polycyclic aromatic hydrocarbon formed in incomplete combustion of organic matter, found in grilled meat and tobacco smoke and metabolised into a carcinogen.
Benzo[a]pyrene activated by CYP → epoxide. Epoxide detoxified by EH → dihydrodiol. Dihydrodiol activated by CYP → benzo[a]pyrene diol epoxide, which is detoxifie. Intermediate conjugated to GST and excreted
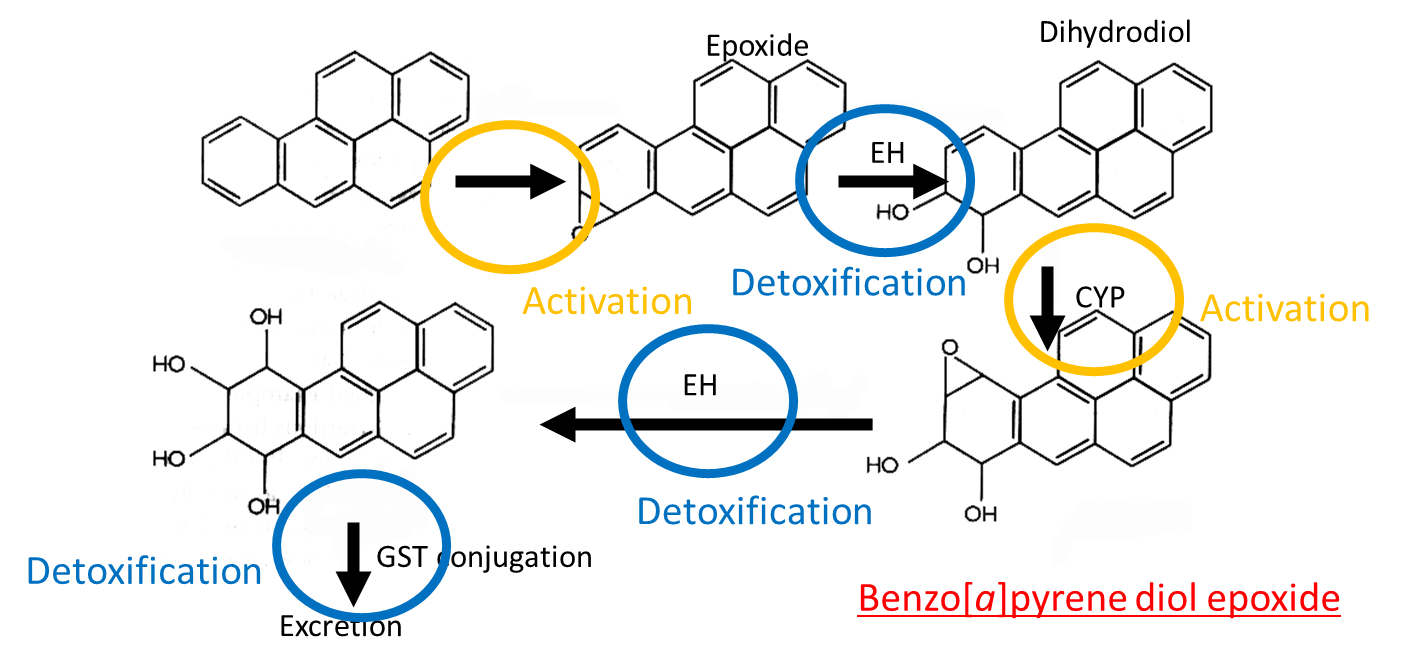
Benzo[a]pyrene diol epoxide is an active carcinogen which intercalates into DNA, reacting with guanine bases and distorting the double helix. This disrupts DNA replication and causes mutation eg in p53 it causes G to T mutation.
Intrinsic factors affecting detoxification enzymes includes species due to different dietary challenges, sex due to hormonal enzyme regulation and diet as it is the main source of xenobiotics.
Genetic polymorphisms are alterations in gene sequence leading to 2+ forms of the allele in the population eg single nucleotide polymorphisms. Alteration in genotype can affect phenotype and gene product activity may be altered. Genetic variation alters susceptibility to toxic substances, causes adverse drug reactions and can increase susceptibility to cancer.
Toxicogenetics is the study of how genetic differences influence susceptibility to toxic effects of chemicals including drugs, environmental toxins and other hazardous substances.
There are generally 4 main genotypes for metabolism: normal, poor, ultrafast and intermediate.
Hepatic CYP2D6 metabolises approximately 25% of prescribed drugs and metabolises codeine to morphine. It is highly polymorphic with over 100 alleles. In poor metabolisers pain relief may be inadequate whereas ultra fast metabolisers have a rapid increase in morphine and may experience overdose symptoms on a therapeutic dose.
Poor metabolisers - CYPD2D6:
0.4 to 6.5% of population
CYP2D6*4 mutation
intron 3/exon 4 boundary
shift of consensus acceptor splice site - mRNA has extra bases and premature stop codon
OR CYP2D6*5 deletion
Ultra-fast metabolisers - CYP2D6:
1 to 2% of individuals
increased copy number - more genes therefore increased mRNA and protein etc
Pharmacogenomics is the study of how genes affect a person’s response to drugs. Aims to understand how genetic variation affects efficacy, dosage and safety of drugs to optimise drug therapy.
Mutagens cause DNA damage eg point mutation, DNA rearrangement, DNA and chromosomal breaks. Mutations in germ cells can give rise to inherited genetic diseases. Mutagens can cause cancer, trigger cell cycle arrest and cell death.
ROS causes lipid oxidation. Lipid oxidation products can damage DNA, resulting in altered membrane dynamics and impaired membrane function. Membrane leakage and cell lysis may occur via ferroptosis.
Protein damage occurs through oxidation of amino acid side chains. Sulphur containing amino acids are at risk. Amino acid oxidation alters protein conformation, potentially affecting function which is a major issue if protein involved in DNA repair, transcription, translation or cell cycle control.
Necrosis = non-regulated cell death. Cell and organelles swell, random DNA degradation and plasma membrane function.
Types of regulated cell death = apoptosis, autophagy, necroptosis, pyroptosis, cuproptosis and ferroptosis.
apoptosis | autophagy | necroptosis | pyroptosis | cuproptosis | ferroptosis |
cell shrinkage, membrane bleb | autophagosome forms | swelling of cell and organelles | cell swells | mitochondrial outer membrane permeabilisation | normal cell size |
unaltered mitochondria | DNA condensation | mitochondria outer membrane swells and becomes permeable | inflammasome forms | DNA condensation, large scale fragmentation | smaller than normal mitochondria, increased density and no cristae |
DNA condensation and fragmentation | large scale fragmentation | random DNA degradation | DNA condensation and fragmentation | no DNA condensation | |
membrane rupture | membrane pores form | no loss of membrane integrity |
/