2.1.2 Carbohydrates
2.1.2 Biological molecules (d-g and part of q)
Carbohydrates - Notes
2.1.2d: the ring structure and properties of glucose as an example of a hexose monosaccharide and the structure of ribose as an example of a pentose monosaccharide
- State the elements present in carbohydrates. (F)
- State the general formula of carbohydrates. (F)
- Define the term “monosaccharide”, “disaccharide” and “polysaccharide”. (F)
- Define the term “pentose sugar” and “hexose sugar”. (F)
- Define the term “triose sugar” and name an example. (S+C)
- Describe what is meant by a “furanose ring” and a “pyranose ring”. (S+C)
- Draw a molecule of alpha-glucose, beta-glucose and ribose.
- Draw a simplified molecule of alpha- and beta-glucose. (F)
- Define the term “isomer”.
- Describe the difference between alpha- and beta-glucose. (F)
- Draw a table listing the properties and differences between alpha-glucose and ribose.
2.1.2e: the synthesis and breakdown of a disaccharide and polysaccharide by the formation and breakage of glycosidic bonds
- List 3 examples of disaccharides and for each state which monosaccharides they are composed of. (F)
- State the properties and functions of glucose, fructose, galactose, maltose, sucrose and lactose. For each also state where they occur.
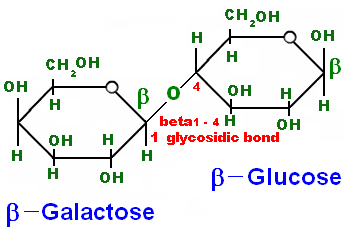
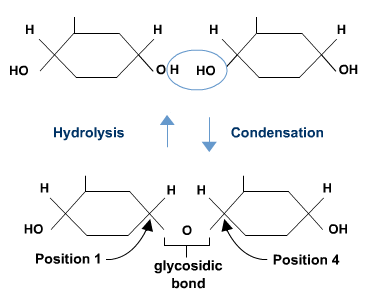
- Draw a labelled diagram demonstrating how two molecules of glucose form a disaccharide in a condensation reaction, showing the location of a 1,4-glycosidic bond and from where a water molecule is generated. (F)
- Describe, using a diagram, how hydrolysis of maltose occurs and why water is needed. (F)
2.1.2f: the structure of starch (amylose and amylopectin), glycogen and cellulose molecules
- Explain why alpha-glucose links together to form starch whereas beta-glucose links together to form cellulose.
- List the two different polysaccharides that make up starch. (F)
- Explain with the use of diagrams why glycosidic bonds are called 1,4 or 1,6.
- Explain how to calculate the number of glycosidic bonds in a carbohydrate given the number of monosaccharides it is made up from. (S+C)
- Explain how to calculate the chemical formula for a carbohydrate given the number of hexose sugars it is made up from. (S+C)
- Describe the structure of a cellulose fibre.
2.1.2g: how the structures and properties of glucose, starch, glycogen and cellulose molecules relate to their functions in living organisms
- Draw a table listing the properties and functions of glucose, starch, cellulose and glycogen.
- Relate the structural properties to the functions of starch, cellulose and glycogen with reference to their solubility, size, bonding and presence of side-chains. (F)
2.1.2q: how to carry out and interpret the results of the following chemical tests: biuret test for proteins, Benedict’s test for reducing and non-reducing sugars, reagent test strips for reducing sugars, iodine test for starch, emulsion test for lipids.
- Draw a table that shows the chemicals used, an outline of the method, the appearance of a negative results and the appearance of a positive result for the biuret test for proteins, the Benedict’s test for reducing sugars, the Benedict’s test for non-reducing sugars, the iodine test for starch and the emulsion test for lipids. (F) [carbohydrate ones only]
- Define the terms “qualitative test”, “semi-quantitative test” and “quantitative test”. (F)
- Explain how the Benedict’s test for reducing sugars can act as a semi-quantitative test (include the colour range that can be seen with different concentrations of glucose).
- List 5 examples of reducing sugars.
- State one example of a non-reducing sugar.
- Explain why reducing sugars are called “reducing” sugars. (S+C)
An introduction to carbohydrates
Carbohydrates contain the elements carbon, hydrogen and oxygen.
The term carbohydrate means ‘hydrated carbon’ and this comes from the fact that the general formula for carbohydrates is:
Cx(H20)y
Terminology
Monosaccharide = a single sugar unit
Disaccharide = a sugar consisting of two monosaccharides
Polysaccharide = a molecule consisting of many monosaccharides joined together in a chain
Pentose sugar = a monosaccharide with 5 carbons in the molecule
Hexose sugar = a monosaccharide with 6 carbons in the molecule
Triose sugar = a monosaccharide with 3 carbons in the molecule
Furanose ring (S+C) = a loop of joined atoms in a monosaccharide consisting of 5 atoms in the ring (4 carbons and 1 oxygen)
Pyranose ring (S+C) = a loop of joined atoms in a monosaccharide consisting of 6 atoms in the ring (5 carbons and 1 oxygen)
Isomer = a molecule that has the same chemical formula as another molecule but a different structural arrangement of atoms.
The diversity of carbohydrates (at A-level)
Properties of the groups of carbohydrate (with examples)
Monosaccharides – e.g. Glucose, Fructose, Galactose, Ribose, Deoxyribose
- One sugar unit
- Soluble in water
- Sweet (a sugar)
- Transport function – e.g. glucose in animals
- Component of nucleic acids – e.g. ribose (RNA) and deoxyribose (DNA)
Disaccharides – e.g. Maltose (Glu+Glu), Sucrose (Glu+Fru), Lactose (Glu+Gal)
- Two sugar units
- Soluble in water
- Sweet (a sugar)
- Transport function – e.g. sucrose in plants, lactose in milk
Polysacchardies – e.g. Amylose, Amylopectin, Glycogen, Cellulose
- Many sugar units
- Less or not soluble in water
- Storage – e.g. starch (amylose + amylopectin) [plants], glycogen [animals]
- Structural – e.g. cellulose
The pentose sugars
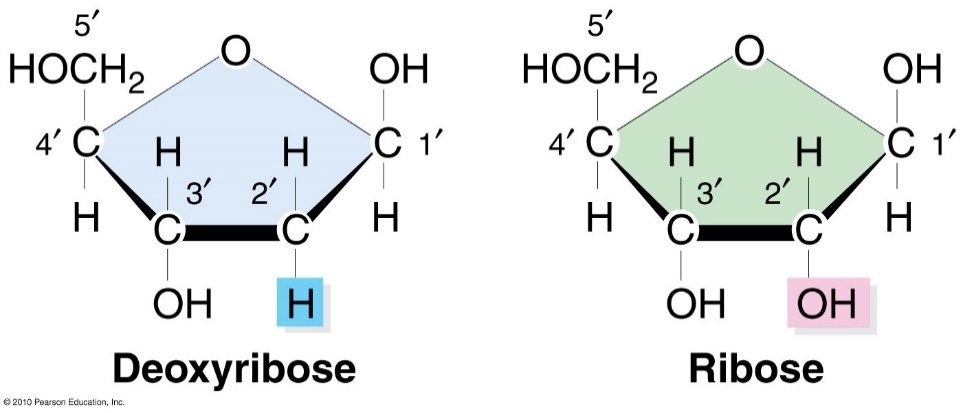
Deoxyribose is found in DNA and ribose is found in RNA
Glucose as an example of a hexose sugar
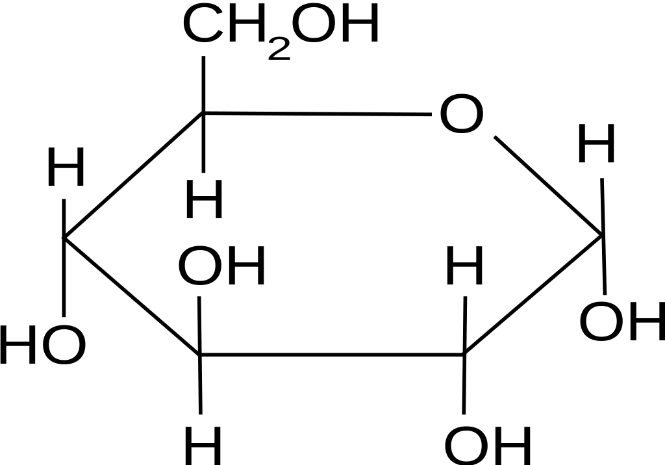
Only the key atoms in glucose are drawn in.
All atoms drawn in expect the carbons in the ring (they are at the 5 points of the hexagon).
All atoms drawn in.
Carbons numbered.
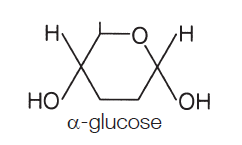
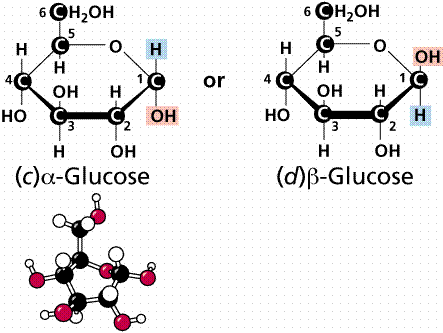
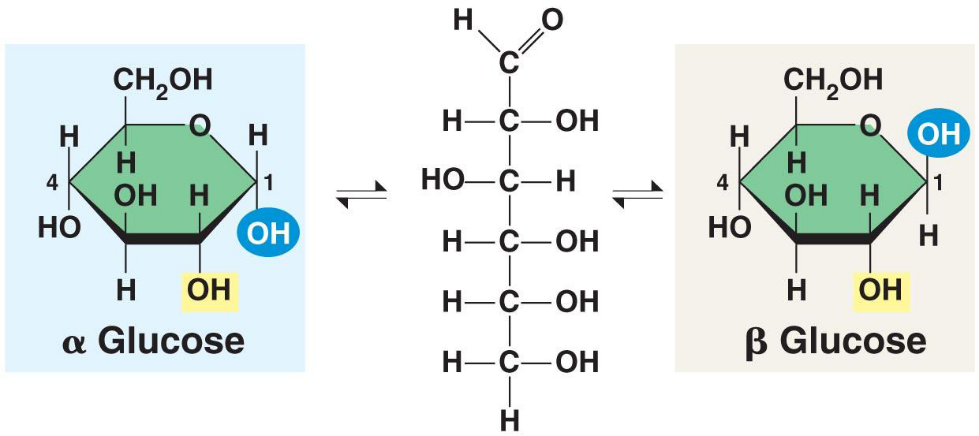 The two forms of glucose
The two forms of glucose
In solution glucose spontaneously flips between a ring formation and a straight chain. When the straight chain becomes a ring there are two different forms the ring structure can take.
When the hydroxyl (OH) group on carbon 1 is below the plane of the ring (on the opposite side to the side chain) the molecule is called alpha-glucose (or α-glucose).
When the hydroxyl (OH) on carbon 1 is above the plane of the ring (on the same side as the side chain) the molecule is called beta-glucose (or β-glucose).
 The structures of the other sugars (S+C)
The structures of the other sugars (S+C)
Fructose
(helps make fruit taste sweet and part of sucrose)
Galactose
(in milk as part of lactose)
Sucrose
(the transport sugar in plants)
Lactose
(in milk)

Maltose
(a step in the digestion of starch, important for seed germination)
Converting 2 glucoses into maltose (and back again)
Reducing and non-reducing sugars and the Benedict’s test
Reducing sugars are those that will allow Cu2+ ions in Benedict’s reagent to be reduced (i.e. gain an electron) to Cu+ ions. Benedict’s reagent starts blue because the Cu2+ ions are blue. If the solution is heated (to near boiling, above 80°C) and a reducing sugar is present the Cu2+ ions become Cu+ ions which aren’t blue and the solution becomes paler until eventually it is colourless. The Cu+ ions bind with oxygen to become copper oxide (CuO) which is insoluble and forms a brick red precipitate.
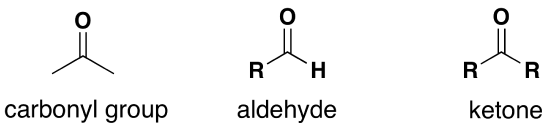 The chemical groups in sugars that allows the reduction of Cu2+ ions are an aldehyde group or a ketone group
The chemical groups in sugars that allows the reduction of Cu2+ ions are an aldehyde group or a ketone group
All monosaccharides, in their straight chain form, have either an aldehyde group or a ketone group and so they are all reducing sugars. Both Maltose and Lactose are also reducing sugars because the glucose whose carbon 4 is involved in the glycosidic bond is still able to spontaneously change into its straight chain form whilst remaining attached to the other monosaccharide.
Neither the glucose nor the fructose in sucrose can change into their straight chain forms and so sucrose doesn’t have an aldehyde or a ketone group. It is called a non-reducing sugar and won’t alter the colour of Benedict’s reagent when heated with it.
If, however sucrose is heated with acid it hydrolyses into glucose and fructose. Once neutralised (because the Benedict’s test only works at a neutral pH) this solution will, of course, now test positive for reducing sugars.
So, a test for non-reducing sugars is to split a sample in two, heat the first sample with Benedict’s reagent and observe, then heat the second solution in acid, allow to cool and then neutralise. Then heat this with Benedict’s reagent and observe. A positive result for non-reducing sugars is no colour change (i.e. the solution remains blue) in the first sample and then a brick red precipitate forming in the second sample.
Types of chemical test
If a chemical test only tests for the presence or absence of a chemical and doesn’t tell you about how much of the chemical is present it’s called a qualitative test.
If a chemical test allows you to determine the concentration of the chemical then it is called a quantitative test.
Some chemical tests allow you to see whether the concentration of one solution is higher or lower than another solution (or allows you to identify a range of concentrations that your solution must fall within) but it doesn’t allow you to put a value to the concentration. These chemical tests are called semi-quantitative.
The Benedict’s test can be used as all 3 types with minor modification.
- It can be qualitative if you are just looking to see whether there is any precipitate or not.
- It can be semi-quantitative by looking at the degree of colour change that occurs (some types of Benedict’s reagent go from green to yellow to orange to red and the concentration of reducing sugar increases)
- It can be quantitative if the ‘blueness’ of the solution at the end is measured using a colorimeter, or if the mass of precipitate formed is measured. For both of these a ‘calibration curve’ needs to be created where known concentrations of reducing sugar are studied to see what value from the colorimeter, or mass of precipitate, equates to what concentration of reducing sugar.
Polysaccharides
Polysaccharides are molecules that are composed of many glucose molecules joined together in a chain using condensation reactions.
How many glycosidic bonds are there in a polysaccharide made of 100 glucose molecules?
What is the chemical formula for a polysaccharide made of 100 glucose molecules?
Because they are so large, all polysaccharides are insoluble in water.
Storage polysaccharides
Amylose, amylopectin and glycogen are all molecules with a storage function. They store glucose in an insoluble, chemically inert form.
Amylose and amylopectin are usually found together and this mixture is called starch. It is the carbohydrate storage molecule in plants.
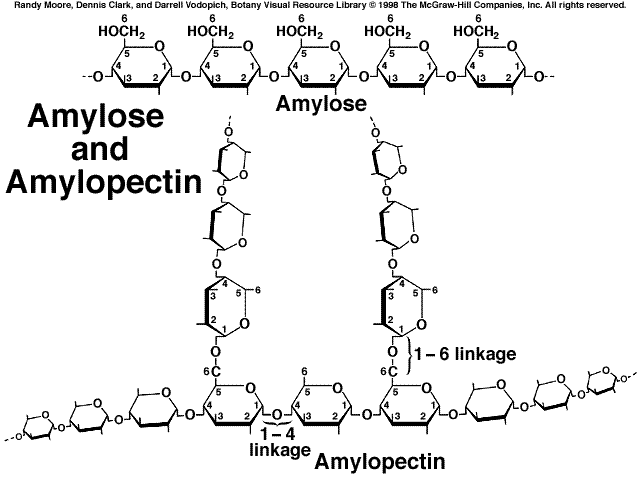
All the storage polysaccharides are made from alpha-glucose.
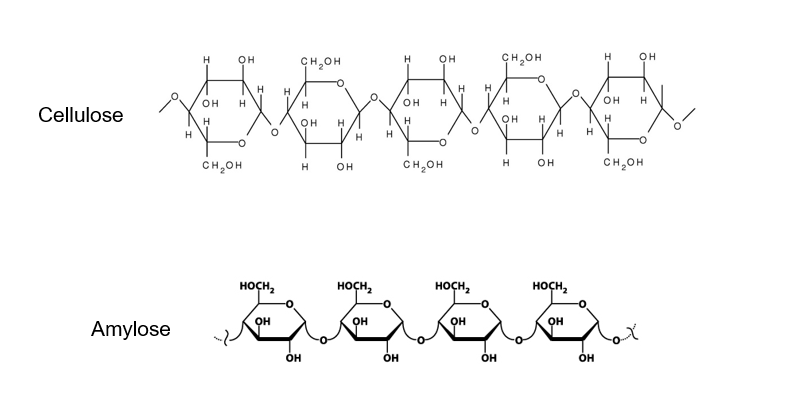 Because the hydroxyl groups on carbon 1 and carbon 4 of alpha-glucose are on the same side of the molecule, the α(1-4) glycosidic bonds that form are all on the same side of the molecule.
Because the hydroxyl groups on carbon 1 and carbon 4 of alpha-glucose are on the same side of the molecule, the α(1-4) glycosidic bonds that form are all on the same side of the molecule.
This causes the molecules to coil and allows them to be a compact store of glucose (i.e. store a large number of glucose molecules in a small space.
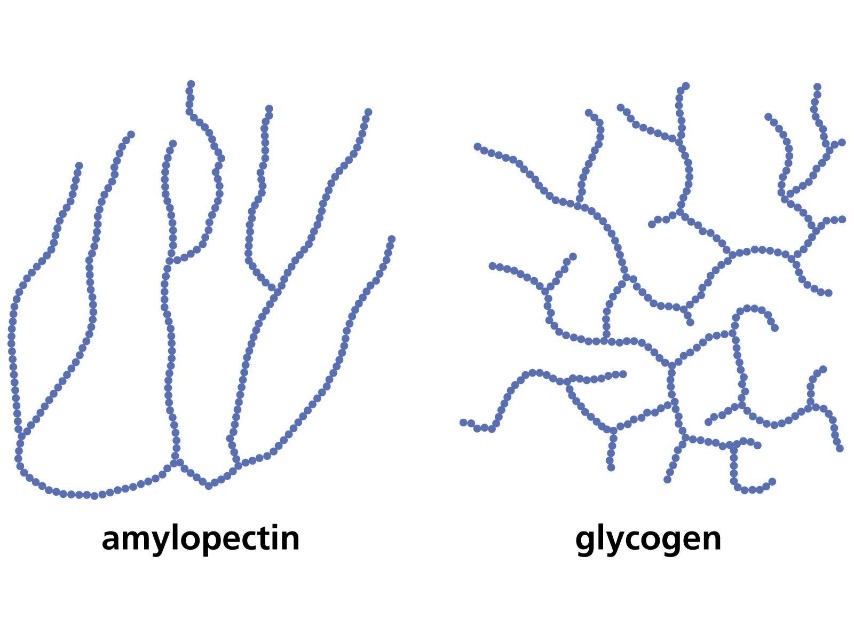 Amylose is just one chain of alpha-glucose molecules joined by α(1-4) glycosidic bonds, however amylopectin and glycogen (the storage carbohydrate in animals) are branched molecules.
Amylose is just one chain of alpha-glucose molecules joined by α(1-4) glycosidic bonds, however amylopectin and glycogen (the storage carbohydrate in animals) are branched molecules.
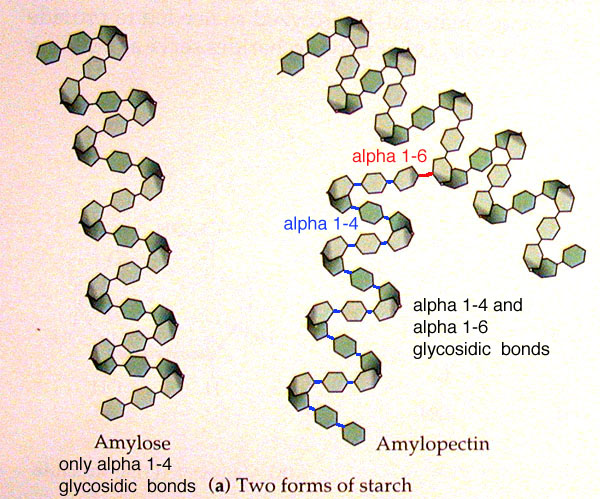 Branches occur due to the formation of α(1-6) glycosidic bonds.
Branches occur due to the formation of α(1-6) glycosidic bonds.
The branches allow for glucose to be released more quickly because the enzymes that hydrolyse polysaccharides do so by binding to the ends of a chain of glucoses. With more branches there are more end points and so more attachment sites for enzymes.
The table below is a summary of how structure relates to function in storage polysaccharides:
Structure | Function |
Large and so insoluble | Doesn’t affect osmosis in cells (i.e. no effect on water potential) |
Coiled | Compact storage – lots of glucose in a small space (and so lots of energy stored in a small space = energy dense) |
Branched due to α(1-6) glycosidic bonds – so there are many end points for enzymes to bind to | Fast release of glucose for respiration |
Relatively inert polymer of alpha-glucose joined by α(1-4) and α(1-6) glycosidic bonds | Acts as a stable store of glucose (i.e. it is metabolically inactive) |
The starch test
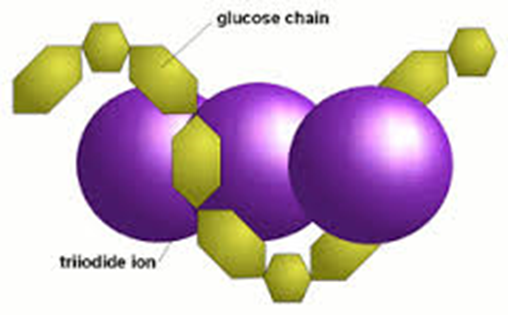 Drop iodine in potassium iodide solution (you can just say iodine) onto/into your sample. If starch is present the solution turns blue-black. If starch is absent the solution remains orange-brown.
Drop iodine in potassium iodide solution (you can just say iodine) onto/into your sample. If starch is present the solution turns blue-black. If starch is absent the solution remains orange-brown.
Part of a starch molecule is a helix (coiled), held in place by weak hydrogen bonds
Iodine solution contains iodide ions, I-
Iodide ions become trapped in the starch helix forming a starch-iodide complex
The starch-iodide complex absorbs a lot of light which is why it looks blue-black
Structural polysaccharides
Cellulose is a structural polysaccharide found in plant cell walls.
It is made from beta-glucose joined by β(1-4) glycosidic bonds and is unbranched.
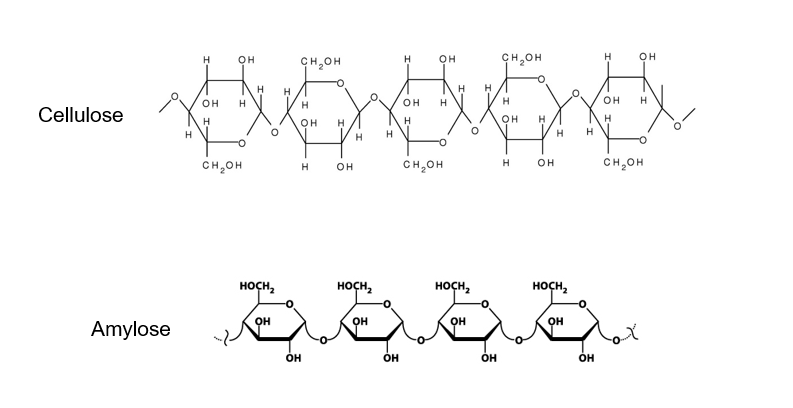 Because the hydroxyl groups on carbon 1 and carbon 4 of beta-glucose are on opposite sides of the molecule, every other glucose molecule must be flipped through 180° so that the hydroxyl groups line up in a way that allow them to form β(1-4) glycosidic bonds. The β(1-4) glycosidic bonds alternate the side of the molecule they are on and so the chain that forms is straight rather than coiled.
Because the hydroxyl groups on carbon 1 and carbon 4 of beta-glucose are on opposite sides of the molecule, every other glucose molecule must be flipped through 180° so that the hydroxyl groups line up in a way that allow them to form β(1-4) glycosidic bonds. The β(1-4) glycosidic bonds alternate the side of the molecule they are on and so the chain that forms is straight rather than coiled.
Cellulose molecules can lie next to other cellulose molecules because they are straight chains. This allows many hydrogen bonds to form between the two molecules.
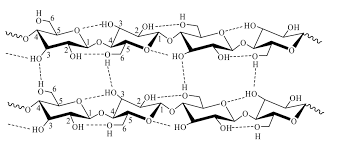
Cellulose molecules combine together in this way to form microfibrils, which combine to form macrofibrils, which in turn combine to form cellulose fibres. This arrangement of cellulose molecules, held together by many hydrogen bonds, gives cellulose fibres very high tensile strength.
Cellulose is also insoluble in water and so retains its physical properties when wet. It is also metabolically inert, meaning that it isn’t broken down easily.
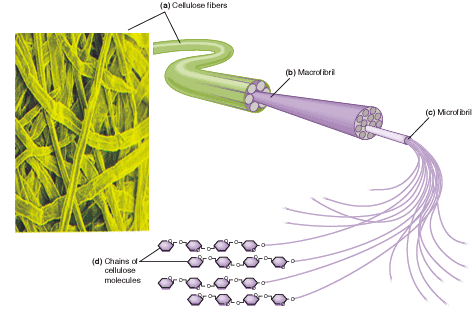
Individual cellulose molecules
Microfibril
Note:
You can call a cellulose cell wall permeable to water because there are large gaps between the fibres however you can’t say that cellulose itself is permeable.
Cellulose fibre
Macrofibril