73 & 74 - Electron configurations I and II
Electron configurations 1
L1 - write electron configurations for elements and identify valence electrons from them
Electron configuration - the distribution of electrons in its atom’s shells and subshells
Aufbau Principle - each electron in a ground state atom fills into the lowest available energy subshell
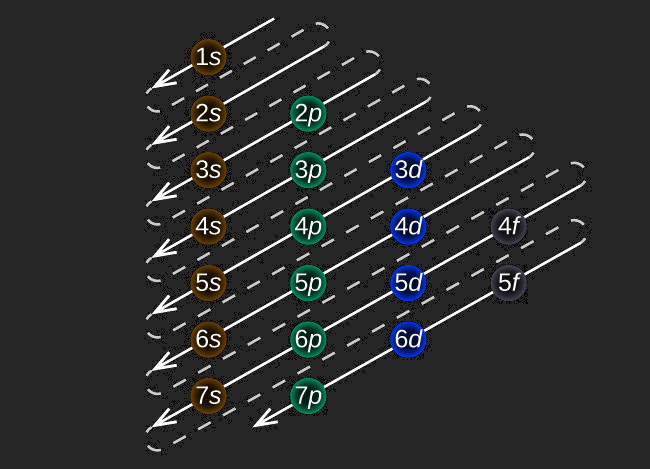
gives the ordering of the subshells
Pauli exclusion principle - no two electrons in the same atom can have exactly the same set of all the four quantum numbers
tells us the max number of electrons in an orbital or subshell
core electrons - the inner shell electrons
have little effect on an elements chemical behavior
valence electrons - outer shell electrons
contribute strongly to its properties and are involved in chemical bonding
we abbreviate the electron configuration of an element using its noble gas core, simplifying the notation while still conveying the distribution of valence electrons.
we use the noble gas that comes before the element we are looking for
ex: the electron configuration for aluminum is 1s2 2s2 2p6 3s2 3p1
the core electrons (1s2 2s2 2p6) correspond ot the noble gas neon so we replace them with the symbol [Ne]
the valence electrons (3s2 3p1) are written nextto the noble gas core, resulting in the abbreviated electron configuration for aluminum as [Ne] 3s2 3p1.
this is called the condensed electron configuration
Electron configurations 2
L1 - write electron configurations for elements and identify valence electrons from them
Orbital diagram
a pictoral representation of an electron configuration
shows individual orbitals
shows pairing arrangements of the electrons
provide more info about electronic structure than electron configurations
require the use of more guidelines
a box represents an orbital
half arrow represents an electron
up arrow indicates an electron with up spin (+1/2)
down arrow indicates an electron with down spin (-1/2)
can use condensed electron configurations when writing orbital diagrams
Hunds rule
in a subshell, electrons first fill separately into each orbital and have the same spin
when each orbital is half filled the second electron fills in and has the opposite spin
Electron configurations of excited atoms
an atom is in an excited state if any of its electrons are not in their (allowed) lowest energy states
if the electron configuration represents an excited atom, it will differ from the sequence corresponding to a ground state atom
Example:
an atom has the electron configuration 1s2 2s2 2p6 3s1 3p6 would this be an excited state electron configuration? what element does it represent?
this electron configuration contains 2 + 2 + 6 +1 + 6 = 17 electrons, which is for an atom so it must represent the element whose atomic number is 17 → chlorine
chlorines ground state configuration → 1s2 2s2 2p6 3s2 3p5
the given electron differs from this showing it would be in an excited state
Anomalous electron configurations
most electron configurations follow the basic rules
for some elements the experimentally determined electron configuration is different
from the predicted configuration due to the stability provided by half-filled or fully filled subshells, leading to exceptions in the expected order of filling.
These exceptions can be observed in elements such as chromium (Cr) and copper (Cu), where the electron configurations are respectively [Ar] 3d5 4s1 and [Ar] 3d10 4s1, instead of the expected [Ar] 3d4 4s2 and [Ar] 3d9 4s2.
 Knowt
Knowt