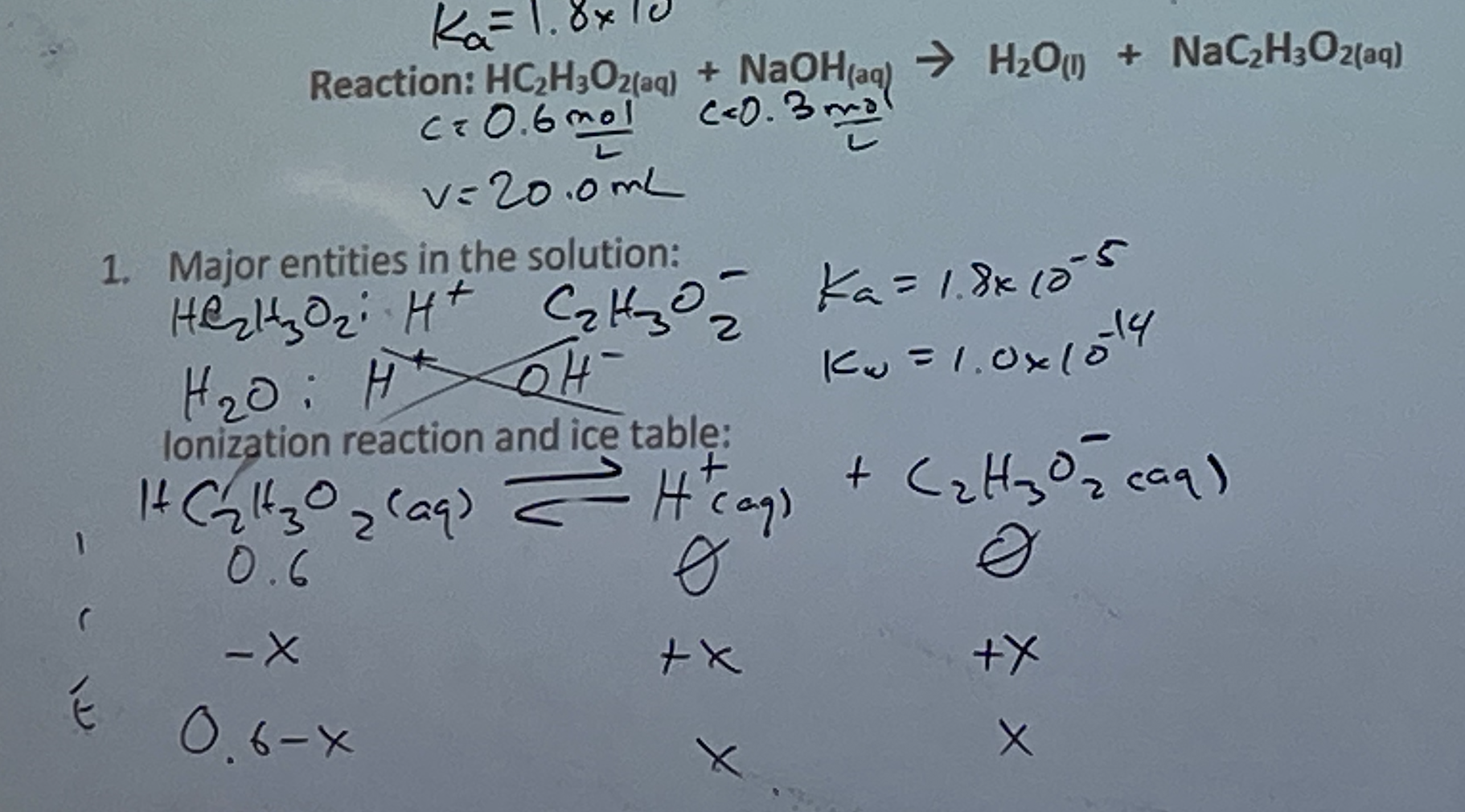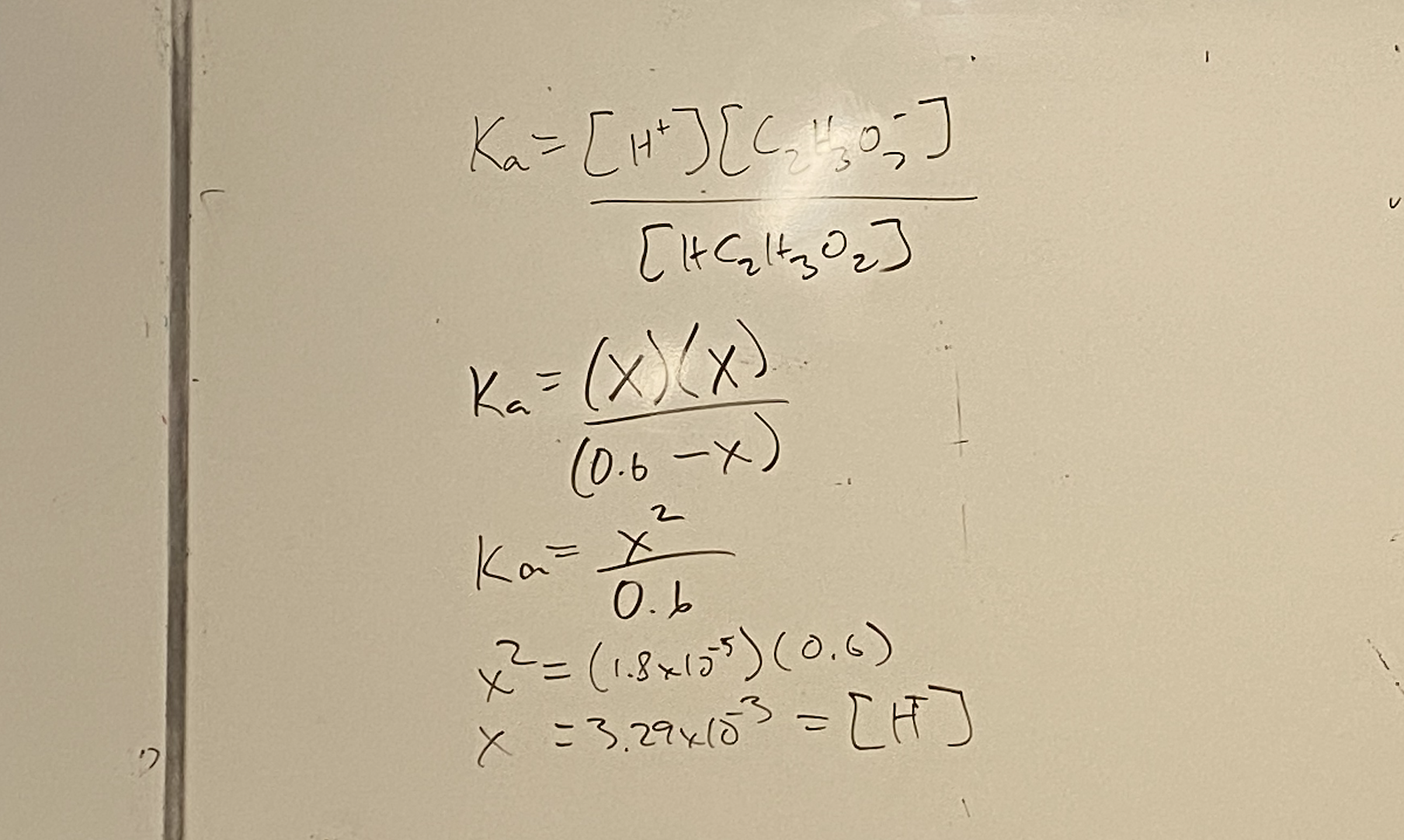8.7 Acid-Base Titrations
Why do we perform titrations?
- used to determine an unknown CONCENTRATION OR pH using a standing solution (solution with known concentrations).
- Acid + Base → Salt + Water
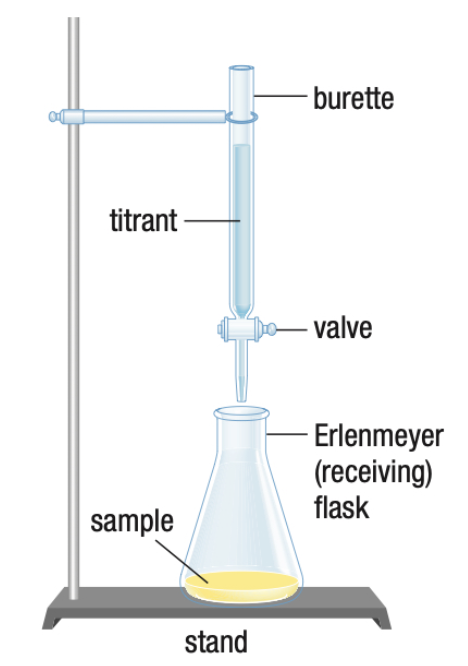
- Titrant: stuff in the burette.
- Sample: stuff in the flask.
- Equivalence point: point where neutralization is complete. [H+] = [OH-]
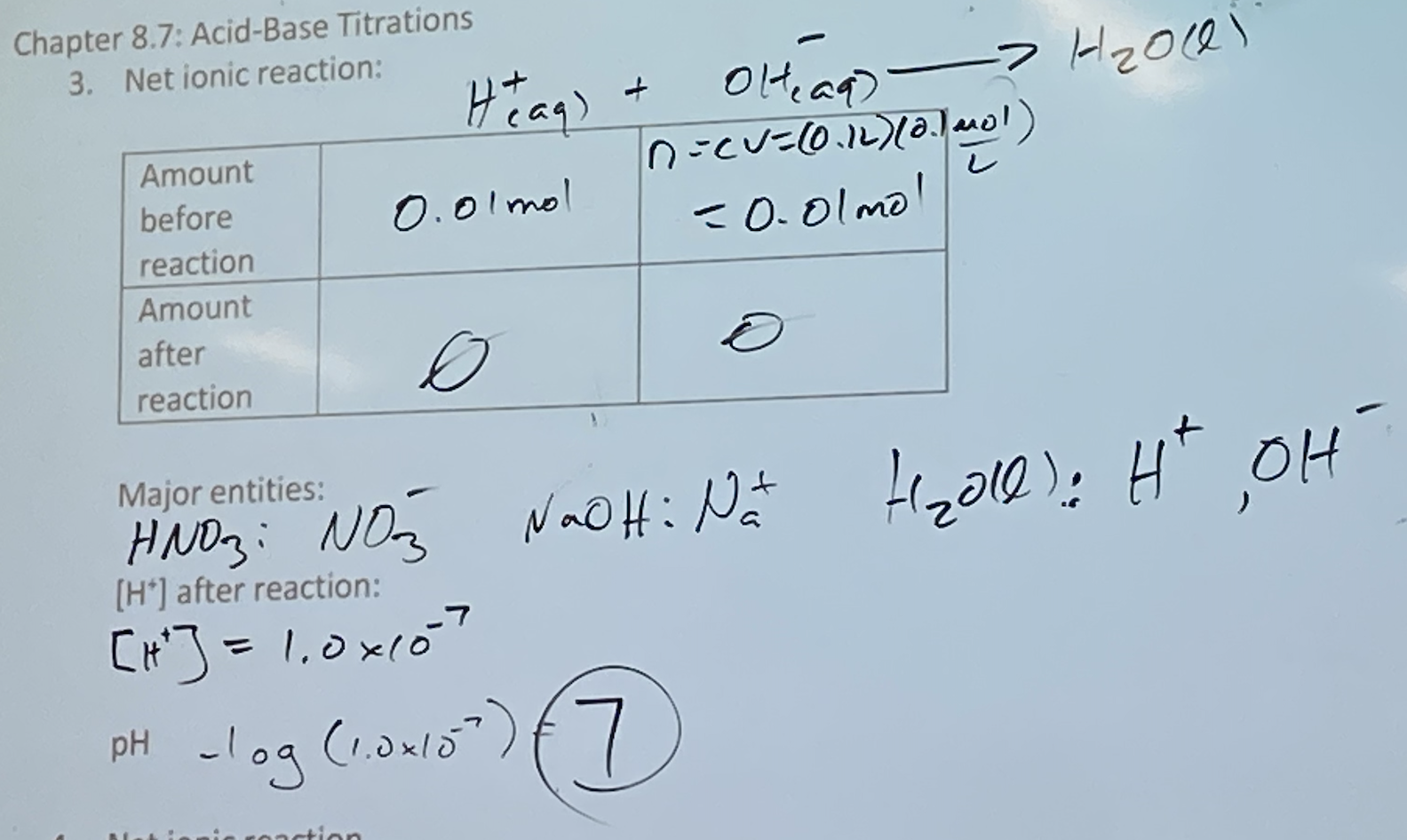
- strong acids and strong bases; pH = 7 in strong/strong; [H+] = [OH-]
- weak / strong; ph does not equal 7; [H+] does not equal [OH-] due to hydrolysis of H2O
- Endpoint: point in a titration where an observable change occurs.
<<Strong Acid, Strong Base: HNO3 (aq) + NaOH(aq) → H2O(l) + NaNO3 (aq)<<


<<HC2H3O2(aq) + NaOH(aq) → H2O(l) + NaC2H3O2(aq)<<
Problem 2: Determine the [H+] and pH of the solution at:
- Before the reaction starts (pH of a weak acid)
- At equivalence point (pH of a salt solution)
- Beyond equivalence point (pH of a strong base)