AOS2
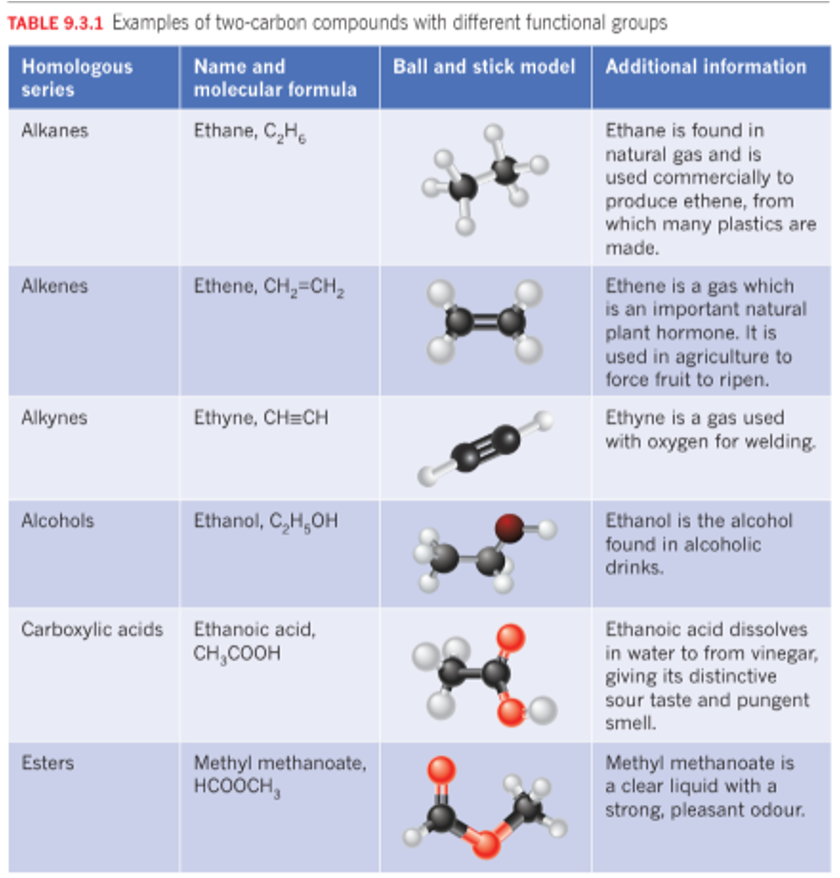
Compounds - are substances that contain two or more different elements.
% of the most abundant isotope is the highest percentage of abundance
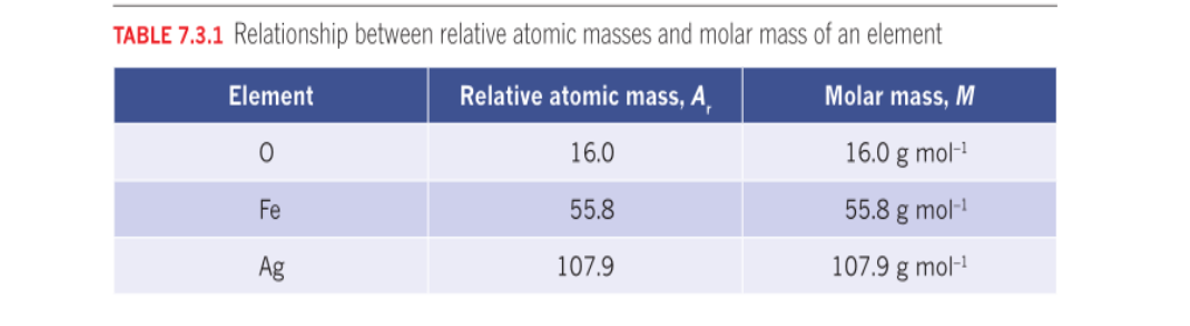
Relative isotopic mass/atomic mass
Definition (BOTH the same)
Relative isotopic mass is the mass of an isotope of an element relative to a standard mass, Carbon-12.
The atomic mass of an element is the relative average of all of the naturally occurring isotopes of that element.
Relative isotopic mass/atomic mass is the mass seen in the periodic table.
Why is Carbon-12 chosen as the standard?
Because it has a mass of exactly 12 grams, and it is a stable and abundant isotope, which means that its mass can be determined accurately.
AzE - the mass number is on top (A), the Z at the button is atomic number, E is the symbol
Why does carbon form more compounds than any other element?
1 carbon atom has 2 × 10-23 grams
Carbon has four valance electrons, so carbon can potentially form covalent bonds with four other atoms.
Strong convalent bonds that could be single, double or tripe bonds
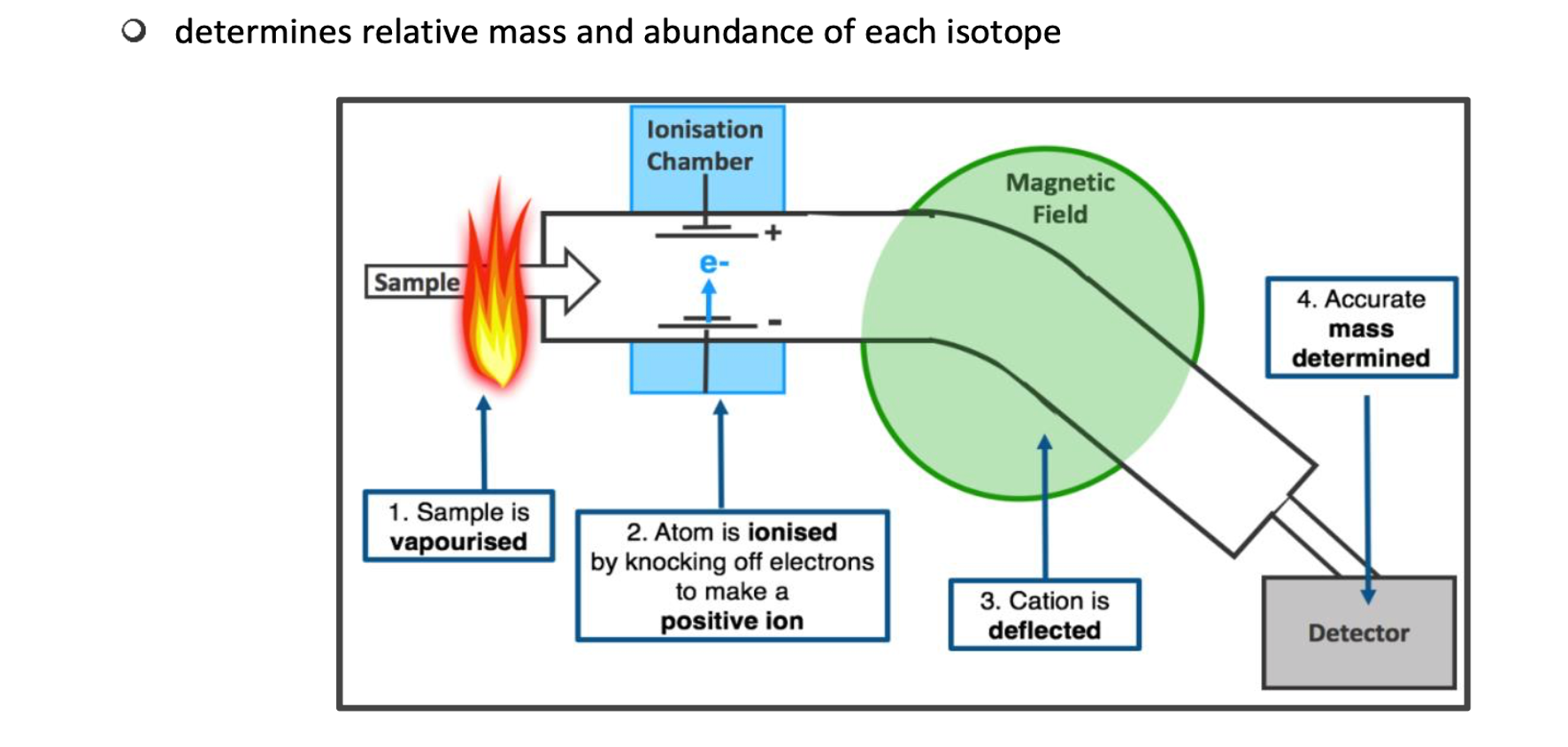
Mass Spectrometer
Instrument that can seperate isotopes of an element based on their mass-to-charge ration, used to determine the relative isotopic mass.
It shows the number of isotopes present in an element, their relative isotopic masses and their proportions
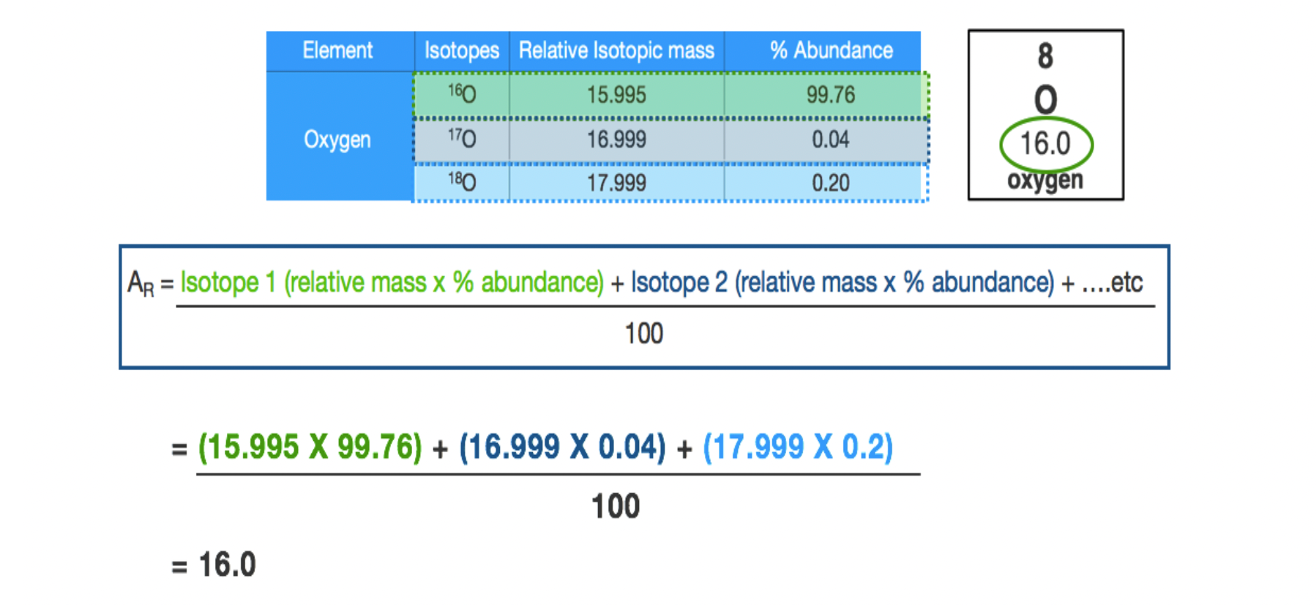
Relative atomic mass (Ar)
Of an element is a weighted average of its isotopic masses.
Calculate the relative isotopic mass formula
Ar = relative atomic mass
![]()
![]()
![]()
Isotopes
Atoms with the same number of protons but different numbers of neutrons.
They share almost the same chemical properties, but differ in mass and therefore in physical properties.
There are stable isotopes, which do not emit radiation, and there are unstable isotopes, which do emit radiation
Do isotopes have the same mass? Why/Why not?
Isotopes don’t have the same mass because they have different numbers of neutrons.
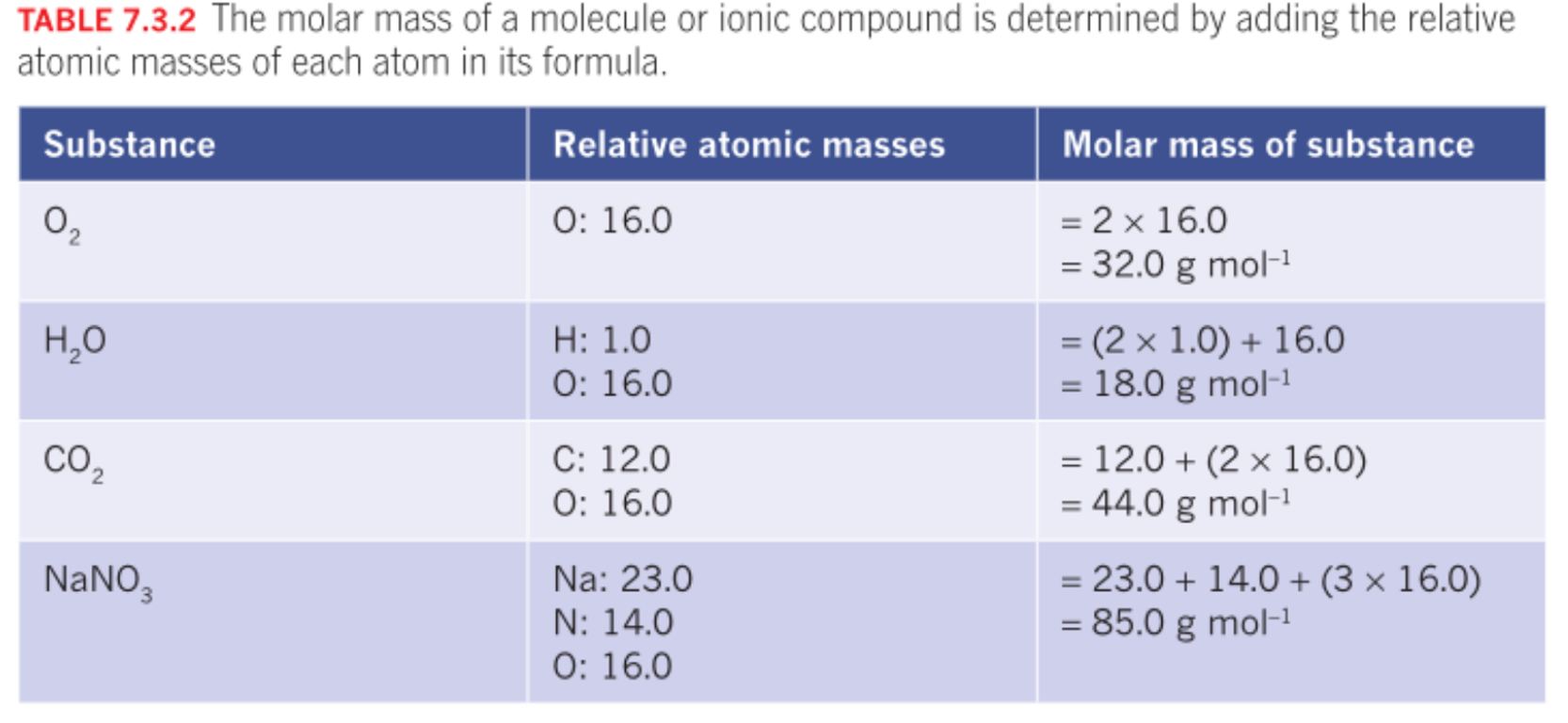
Relative Molecular mass (Mr)
Adding all the relative atomic masses of each atom in a molecule. g mol -1
Mole
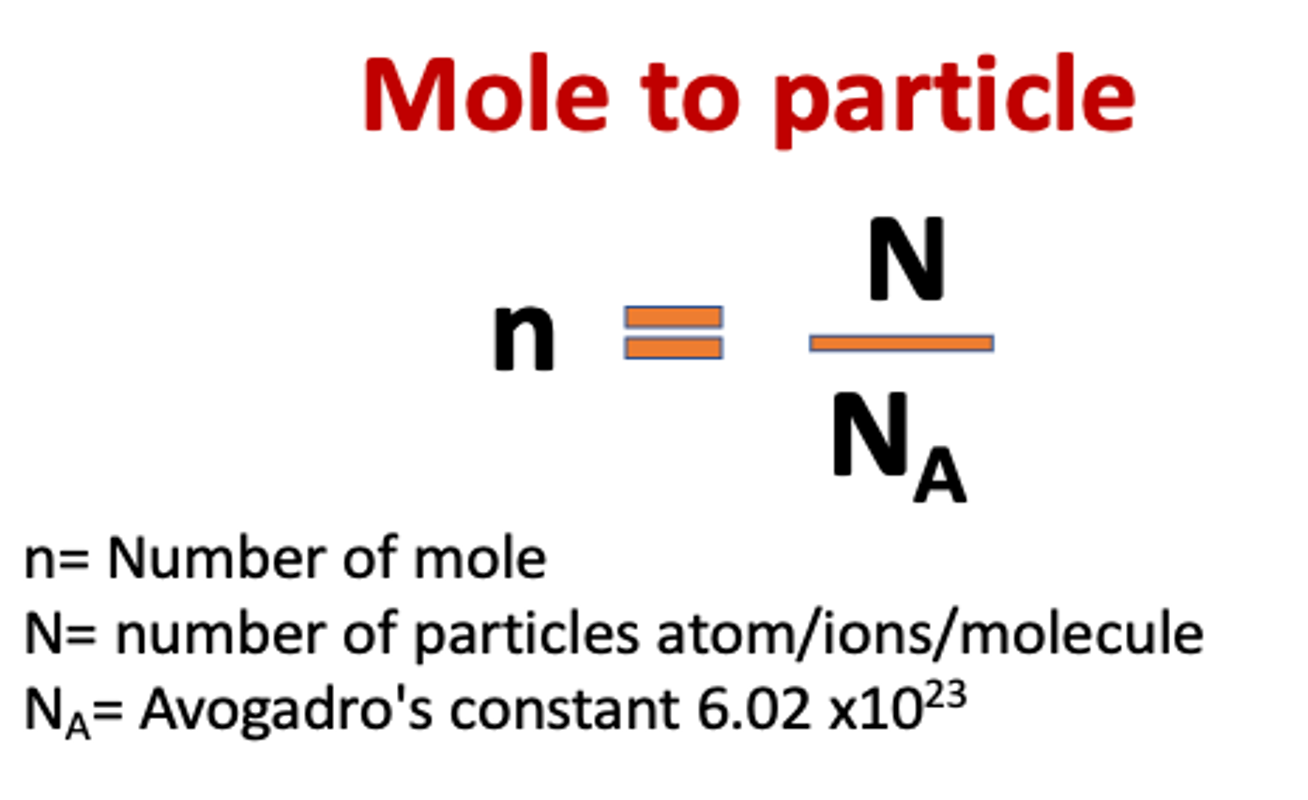
grouping unit that is used to measure the amount of substance
and is given the symbol n, and the unit mol
is referred to as Avogadro’s constant
1 mole of (atoms, molecules or particles) contains particles 6.02 × 1023
n (glucose) = 2 mol (is read as ‘the amount of glucose molecules is 2 moles)
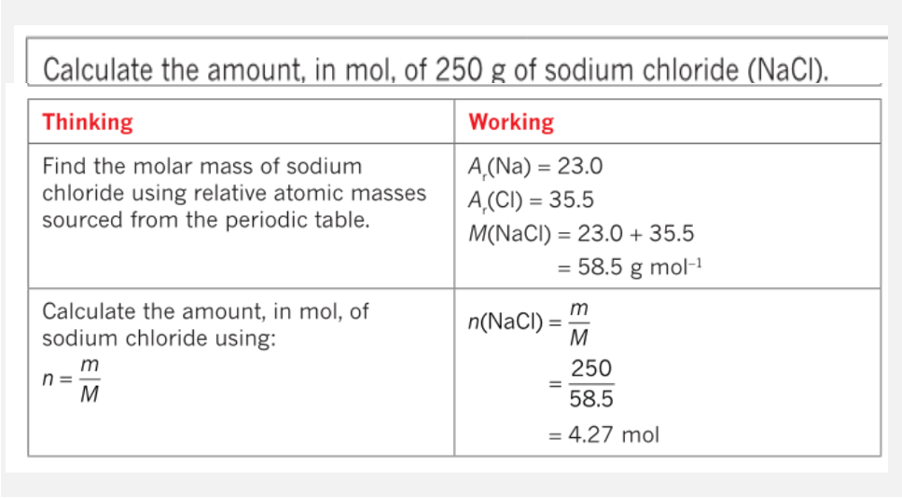
Calculating involving mass and moles
![]()
![]()
Percentage composition

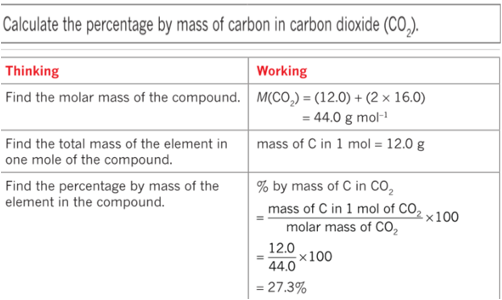 Empirical formula
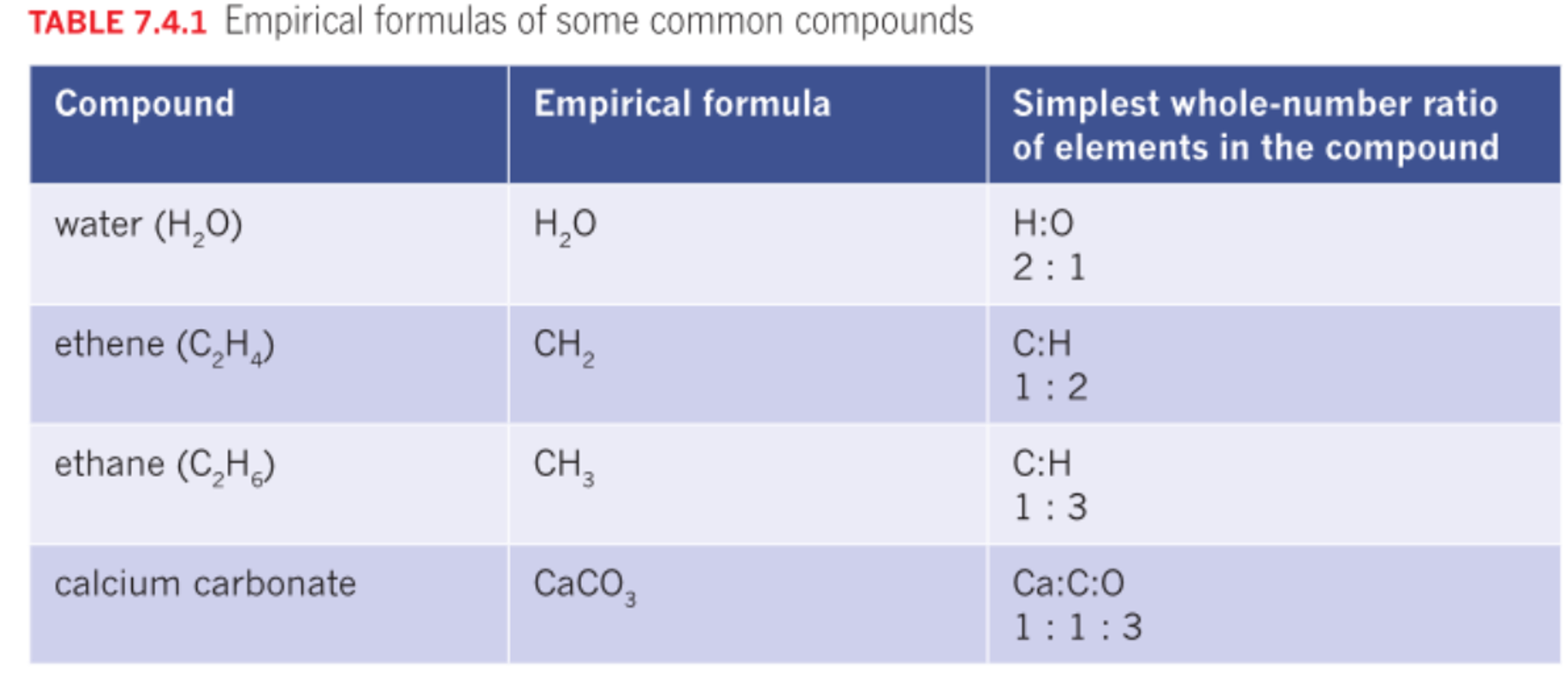
Empirical formula
Simplified whole number ratio of each type of element in the compound.
Ionic compounds only have an empirical formula
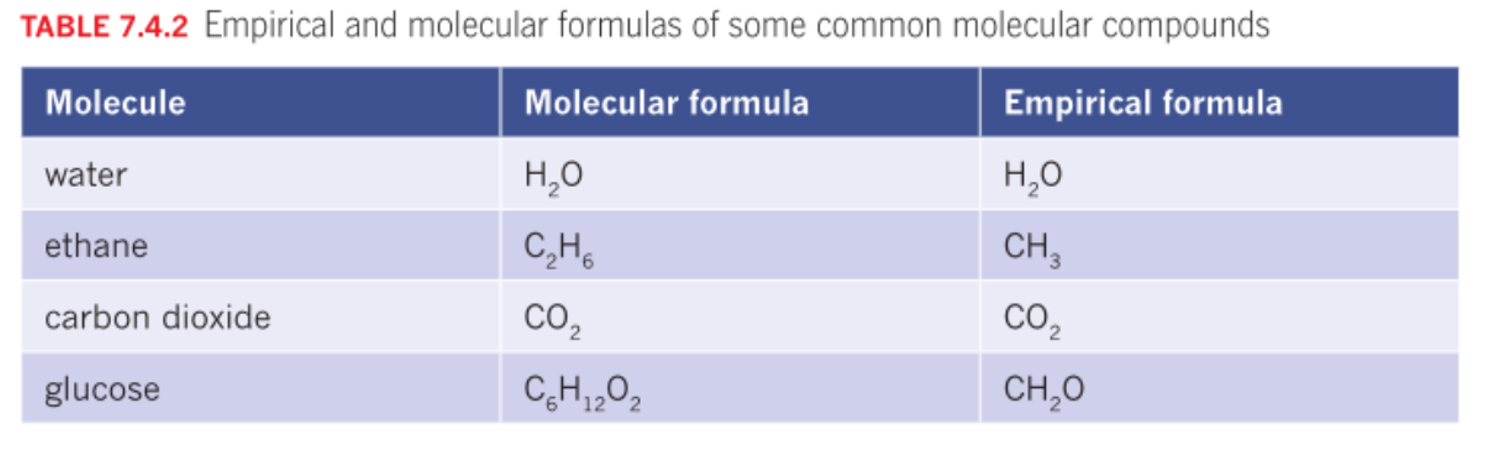
Molecular formula
The actual number of atoms of each element in a compound. (it can be the same as the empirical formula)
Organic and inorganic compounds
Definitions
Organic compounds are only thought to be produced by living or once-living material. Organic compounds are made of carbon, hydrogen, and oxygen.
Inorganic compounds are not carbon-containing compounds and they come from non-living systems.e.g sodium chloride
Examples of organic compounds
They can be found inside and outside the body and may contain other elements.
Examples include caffeine, plastic, petrol, fats, proteins, and carbohydrates. Crude oil and plant-based biomass are sources for organic compounds.
Crude oil
Decaying prehistoric marine microorganisms became part of Earth's crust. Over time high temperatures and pressures formed crude oil.
Crude oil undergoes fractional distillation at refineries to separate its various hydrocarbons based on boiling points
Non-renewable can not be replenished at the rate of use. Finite availability means it is in limited supply and will eventually be used up.
These components are mostly used for fuels, such as gasoline and diesel.
Example of a Fossil fuel
Fractional distillations
This process separates the different types of hydrocarbons in crude oil, based on their boiling points.
The hydrocarbons with longer chains have stronger intermolecular forces, so they have higher boiling points.
Crude oil obtained from this process is used for fuel 90% of the time.
Gasoline (C5 to C12) is obtained from the gasoline fraction, while diesel fuel can be made from either the kerosene (C12 to C16) or diesel/gas oil (C16 to C20) fractions.
Biomass
Organic material from living or recently living organisms.
It can be used for fuel or raw materials.
Used to generate electricity, heat, biofuels, and other products.
Examples of biomass sources include wood, crop residues, and municipal waste. Helps reduce reliance on fossil fuels and fight climate change.
Plant sourced biomass
Plant-sourced biomass refers to carbon-based material that has come from plants.
Plants can offer an alternative renewable source of organic chemicals.
They can intake Carbon dioxide from the atmosphere into glucose via photosynthesis.
Polyethene from sugar
Polyethylene is a common plastic used in consumer products and packaging. It is typically made from petroleum, which is non-renewable.
Bio-based production can convert sugar into polyethylene.
The process involves fermenting sugar to produce ethanol, which is dehydrated to form ethylene, the building block for polyethylene.
Bio-based polyethylene is chemically identical to petroleum-based polyethylene, but it is derived from a renewable resource.
It is a growing alternative to traditional plastics as consumers and companies become more interested in sustainability.
Polyethylene (polyethene) itself is not renewable because it is derived from petroleum, which is a non-renewable resource. However, there are efforts to produce polyethylene from renewable sources such as sugarcane or cornstarch, making it partially renewable in those cases. These renewable sources can be used to create bio-based polyethylene, which still retains many of the properties of traditional polyethylene but has a lower environmental impact due to its renewable origin.
Biofuels
Renewable - can be replaced/replenished at the rate of use
Non-renewable - can’t be replaced
Hydrocarbons
Definition
Hydrocarbons are organic molecules that are covalently bonded and only contain hydrogens and carbons.
Can form chains of varied lengths with different properties and applications.
Types of hydrocarbons
Alkanes: Single bonds between carbon atoms.
Alkenes: At least one double bond between carbon atoms.
Alkynes: At least one triple bond between carbon atoms.
Properties
Nonpolar molecules.
Insoluble in water.
Combustible.
Uses
Uses:
Fuel for vehicles.
Raw materials for plastics, solvents, and lubricants.
Environmental impact:
Combustion releases carbon dioxide, contributing to global warming.
Oil spills can harm marine life.
Sources
Natural gas.
Petroleum.
Coal.
Crude oil
Prefix for the Number of Carbons
Meth-: one carbon.
Eth-: two carbons.
Prop-: three carbons.
But-: four carbons.
Pent-: five carbons.
Hex-: six carbons.
Hept-: seven carbons.
Oct-: eight carbons.
Non-: nine carbons.
Dec-: ten carbons.
Homologous Series Compounds Outline
Definition
Compounds with the same functional group.
Gradual change in physical properties with increasing molecular size.
Characteristics
Similar chemical properties.
Same general formula.
Show a trend in physical properties.
Examples
Alkanes: Methane, Ethane, Propane.
Alkenes: Ethene, Propene, Butene.
Alcohols: Methanol, Ethanol, Propanol.
Physical Properties
Boiling point increases with molecular size.
Solubility in water decreases with increasing carbon chain length.
Density increases with molecular size.
Chemical Properties
Similar reactivity due to the same functional group.
Follow similar reaction mechanisms.
Exhibit similar types of reactions.
Homologous series
Compounds that have a similar structure, same general formula, similar chemical/physical properties
Compounds where each successive member differs only by CH2 from the previous number.
Patterns to their physical properties
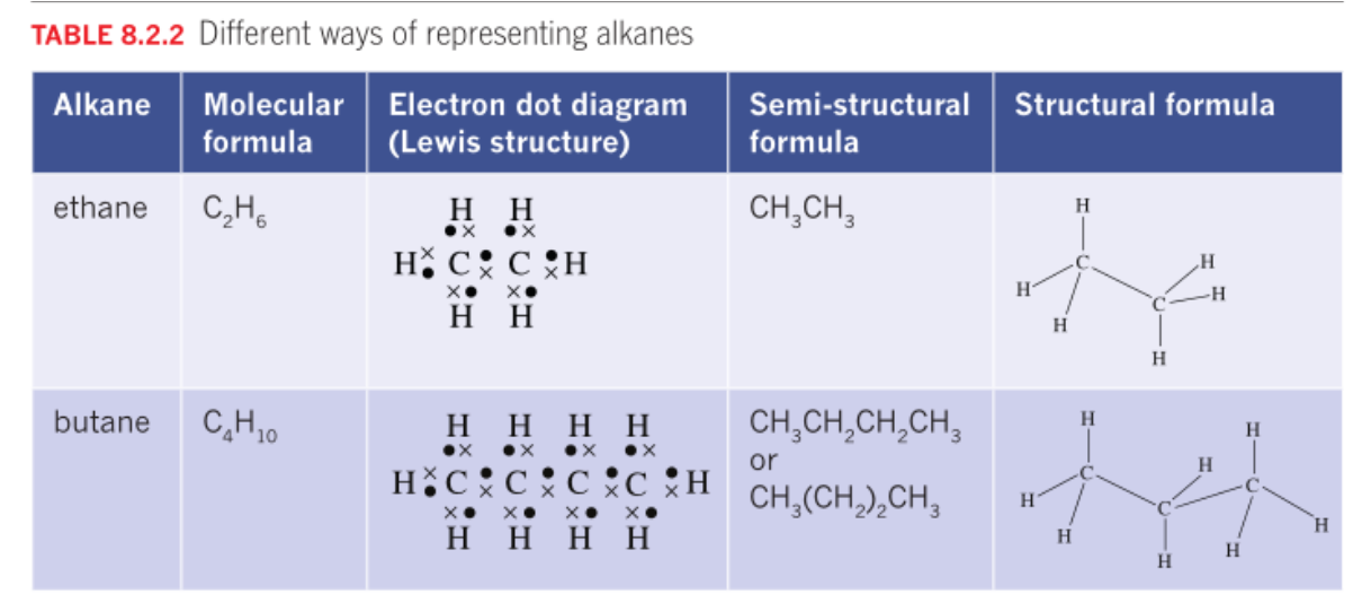
Alkanes
Definition
Saturated hydrocarbons that contains molecules in which all the carbon-carbon bonds are single convalent bonds.
General Formula
CnH2n+2
Suffix "-ane"
Physical Properties
Non-polar - Insoluble in water, the only attractive forces between the molecules are dispersion forces.
Increase in size and number of carbons, increases dispersion forces, increases melting and boiling points
Low boiling points
Chemical Properties
Combustion to produce CO2 and H2O
Alkanes can undergo combustion reactions.
Combustion is the reaction between a fuel and oxygen releasing a significant amount of energy.
Complete combustion is when the supply of oxygen is plentiful, the products of combustion will be carbon dioxide and water.
Alkanes are relatively unreactive, although they do undergo combustion reactions.
Types
Straight-chain alkanes
Branched-chain alkanes
Cycloalkanes
Uses
Fuel (gasoline - used for vehicles and fuel for heating and cooking in households)
Feedstock
Why are alkanes non-polar?
Because the electronegativity difference between the carbon and hydrogen bond is small and so the electrons would sit equally shared between the atoms. And this non-polar nature extends to the molecule as there is only C-H bonds in alkanes.
Alkenes
Definition
Carbon forms many compounds with hydrogen in which there are double bonds between carbon atoms. unsaturated and alynes unsat
General Formula
CnH2n
-suffix: ‘ene’
Physical Properties
Colorless and odorless
Insoluble in water, Non-polar (can’t dissolve in water)
Less dense than water
The longer the carbon chain the more dispersion forces. This means more energy is needed to break the bond.
As chain length increase so does melting and boiling points.
Chemical Properties
Complete combustion
Undergo addition reactions
Can be hydrogenated to form alkanes
More reactive than alkanes
Uses
Production of plastics
Manufacturing of synthetic rubber
Starting material for various organic compounds
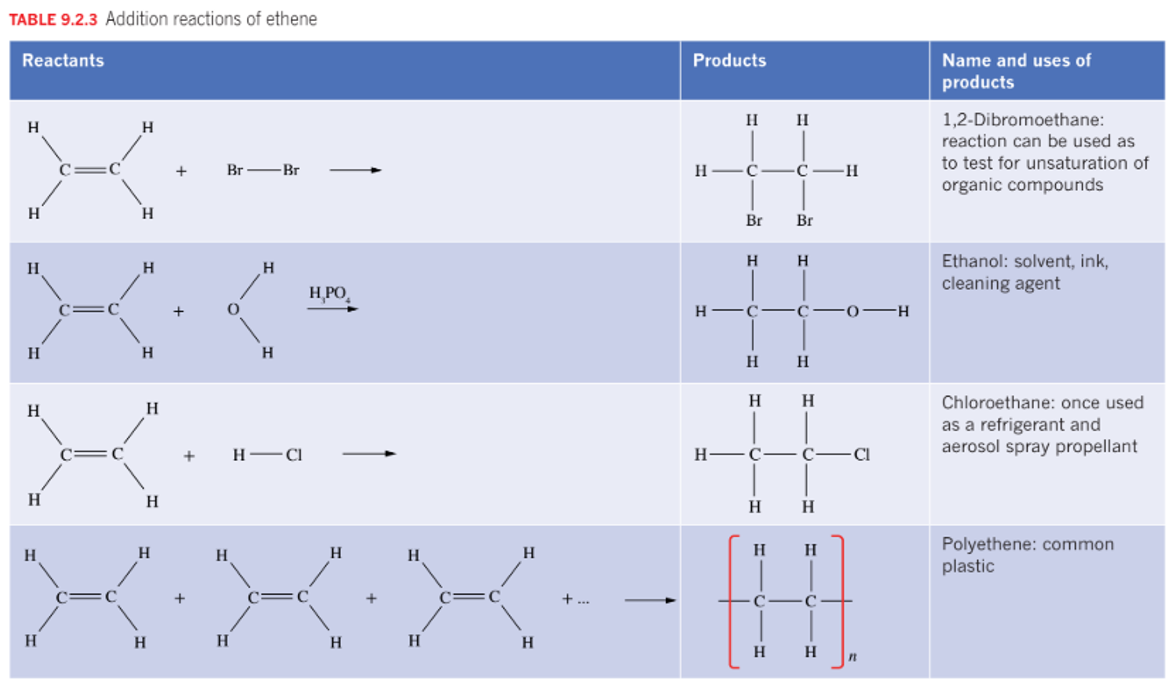
Alkenes undergo two main reactions:
Combustion reactions
Addition reactions
involving them burning with oxygen to produce water and carbon diocide
Ethene + Oxygen → Carbon Dioxide + Water
The reason why alkenes are more reactive than alkanes In addition to reactions, the double bond is split to incorporate the other reactant, and a single C-C bond is formed.
Alkynes
Definition
Unsaturated hydrocarbons with at least one carbon-carbon triple bond
General Formula
CnH2n-2
Physical Properties
Insoluble in water, Polar
Less dense than water
Lower boiling points compared to alkanes
Chemical Properties
Addition reactions
Combustion reactions
Uses
Starting material for organic synthesis
Used in the production of polymers
Used as solvents and in chemical reactions
Cycloalkanes
Not alkenes as they have no double bonds
Cn H2n
Unsaturated hydrocarbon
double or triple carbon-carbon bond in its structure.
Isomers
Definition - Molecules with the same molecular formula but different structural arrangements.
Structural isomers
Structural Isomers
When two molecules have the same molecular formula but different molecular structures
Differ in the bonding sequence of atoms.
Different physical and chemical properties.
Molecules get bigger, the number of structural isomers increases
Functional groups
Carbon forms a covalent bond with other atoms/groups of atoms replacing a hydrogen bond.
Functional group changes physical and chemical properties.
Haloalkanes, Alcohol and Carboxylic acids
alkyl groups: methane → methyl '(yl)’
Steps for Naming Hydrocarbons
Identify the longest carbon chain
Determine the parent name
Use the number of carbon atoms in the longest chain to identify the parent name (meth, eth, prop, but, etc.).
Add the suffix -ane for alkanes, -ene for alkenes, or -yne for alkynes (include the position, use the carbon with the lowest number attached to the bond)
Number the carbon atoms
Start numbering carbons from the end that gives the functional group OR halogens the lowest possible numbers.
Identify functional groups
Identify and name any branches attached to the main chain.
Use prefixes like methyl, ethyl, propyl, etc.
Position
List the functional groups in alphabetical order, ignoring any prefixes.
Combine the names
Write the functional group names followed by the parent name.
Use hyphens to separate numbers and letters in the name.
Use commas to seperate numbers
If your stating a position more than once u also include the position 1 (1-methylprop-1-ene)
but if u only have 1 as a position without any other position then u don’t need to include 1 (butanoic acid)
IUPAC Order of Functional Groups Priority (numbering)
carboxyl - COOH
hydroxyl - OH
amino - NH2
Alkene ( double bond)
Alkyl
Halogens
Properties of Hydrocarbons
Physical Properties
Colorless and odorless
Insoluble in water
Less dense than water
Flammable
Boiling Points
First four are gases at room temp (methane, ethane, propane, butane)
After four they are liquid at room temp
More atoms, more dispersion ( more interaction between molecules), they are more likely to be a liquid at room temp
Flash point
Increase size = increase flash point
Flash point is the lowest temp at which a liquid forms sufficient vapour to ignite.
Meaning a liquid needs to become a vapour so that it can be set on fire.
Depends on how easily it’s going to become a vapour, the lower the temp more flammable.
Substances with larger molecules tend to have higher flash points because their molecules are heavier, more intermolecular forces and require more energy to escape as vapor.
Viscosity
Increase size = increase viscosity ( thickness)
Long chains tangle together
Larger molecules or longer chains in a liquid increase its viscosity because they tend to tangle together and have stronger attractions between molecules, hindering the flow of the liquid. Higher viscosity liquids are thicker and flow more slowly.
Uses
Fuel for vehicles
Raw materials for plastics and polymers
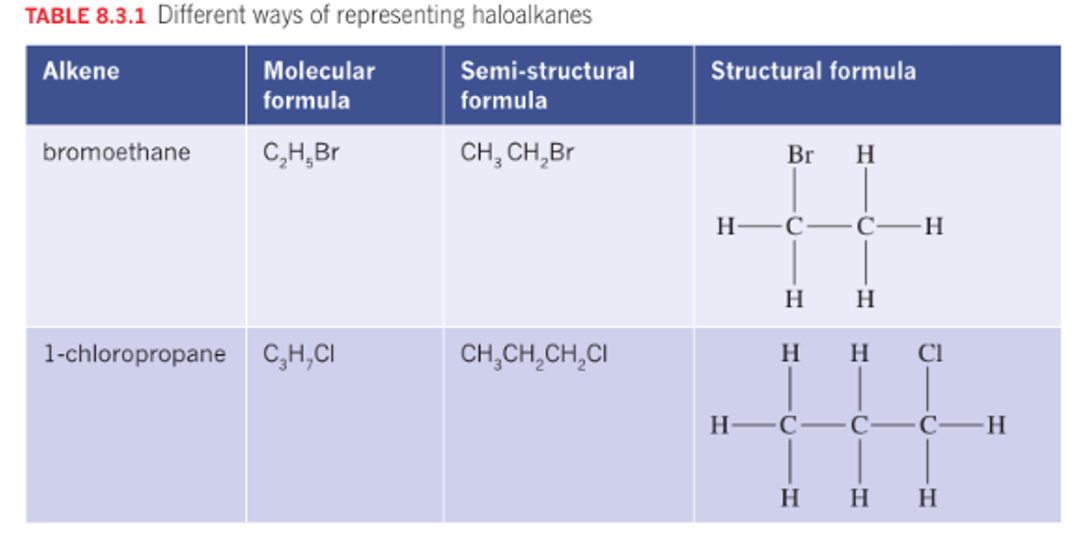
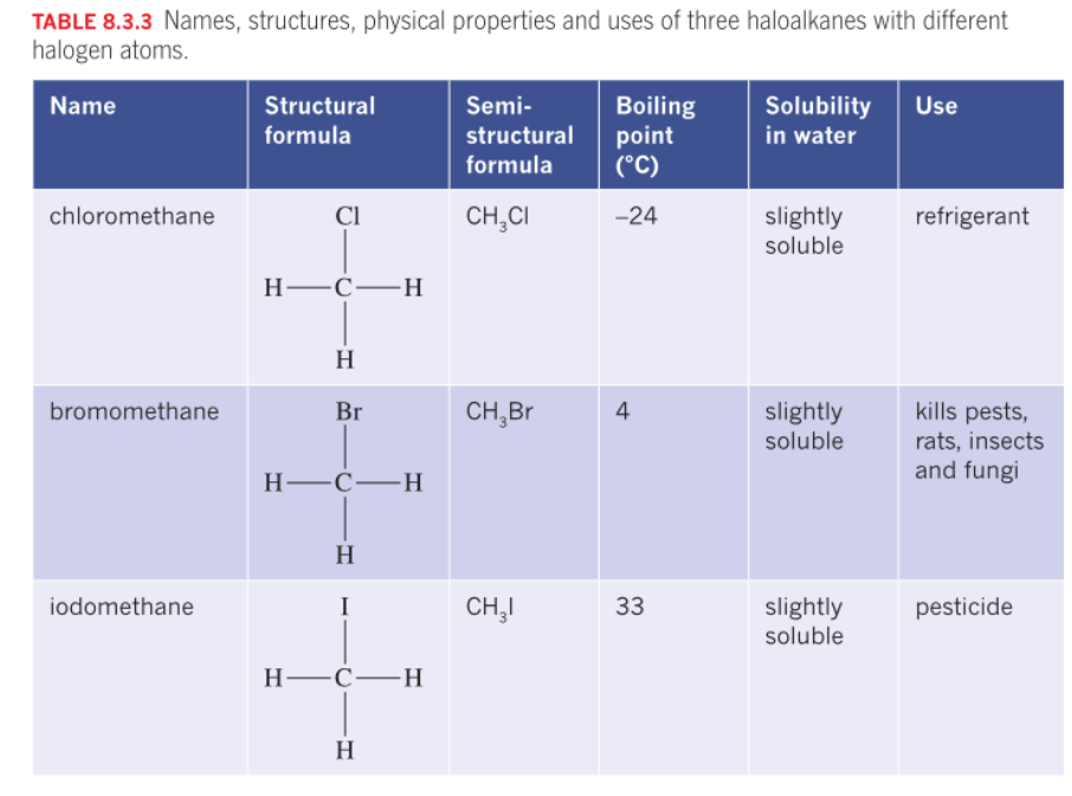
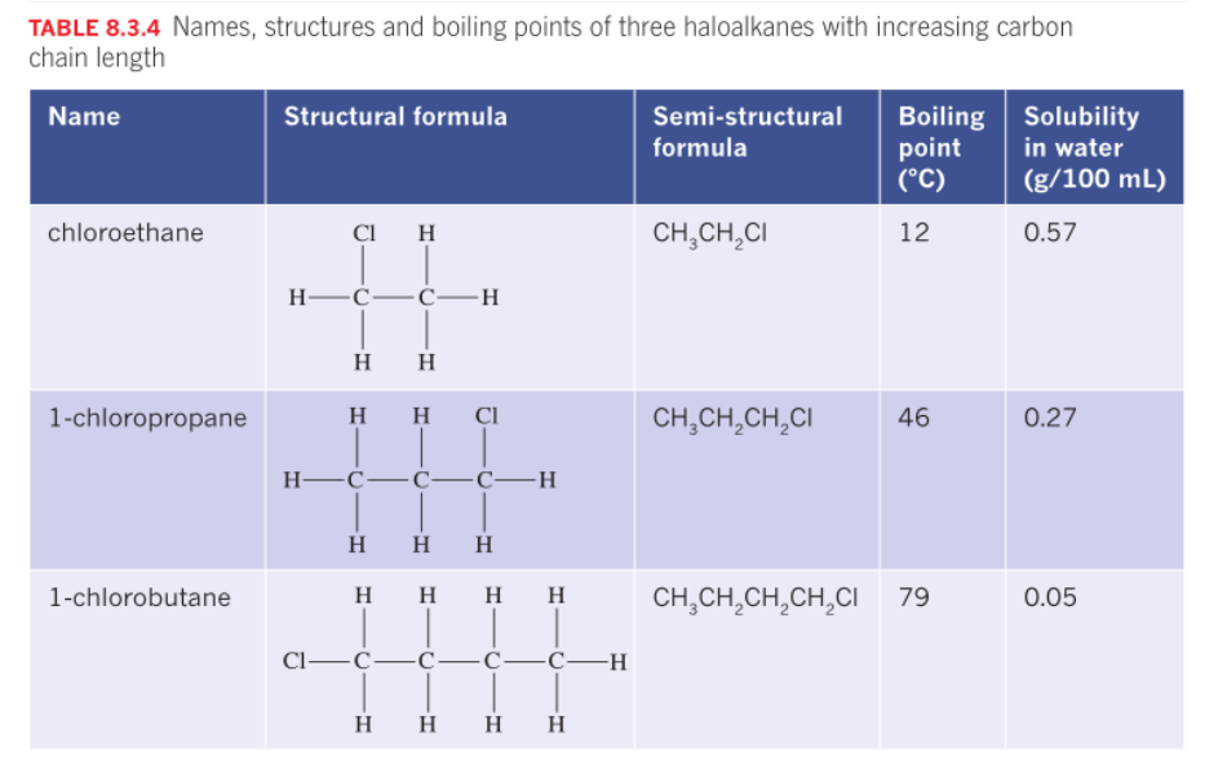
Haloalkanes
Polarity depends on the molecules, but mostly polar.
If the chain is longer it increases BP and MP due to increased dispersion forces.
The solubility decreases as the chain length increases, due to the influence of the non-polar part of the molecule.
The presence of halogen introduces dipole - dipole interactions further increasing energy.
Halogen atoms, such as fluorine, chlorine, bromine, and iodine, are highly electronegative. When they are part of a molecule, they can strongly attract electrons towards themselves, creating a partial negative charge (δ-) on the halogen atom and a partial positive charge (δ+) on the rest of the molecule.
Hydrocarbons with one or more hydrogen atoms are replaced by group 17 Halogens. Formula CnH2n+1 X. X= halogen
fluorine - fluro, chlorine - chloro, bromine - bromo - , iodine iodo
Use: refridgerants
 Chemical properties
Chemical properties
The polarity of Carbon-halogen changes chemical reactivity
Adding halogens alters polarity and how a chemical will react with another.
This means alkanes are more reactive with a halogen attached. Undergo substitution reactions.
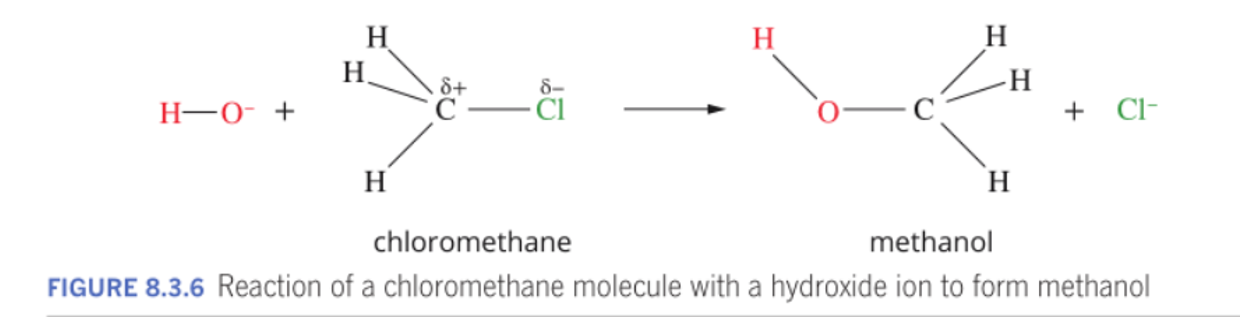 The halogen-carbon bond is polar, making the haloalkane molecule polar in many cases, with dipole-dipole attractions between the molecules.
The halogen-carbon bond is polar, making the haloalkane molecule polar in many cases, with dipole-dipole attractions between the molecules.The boiling points of haloalkanes are higher than for alkanes of the same carbon chain lenth,
Haloalkanes are more reactive than alkanes.
Haloalkanes undergo substitution reactions, where the halo functional group is replaced by another functional group, such as -OH.
Alcohols
OH group, hydroxyl group
It replaced a H in the hydrocarbon, they are polar
suffix ‘ol’
USE: Used as fuels on its own or mixed with petrol
Ethanol can be used as a fuel on its own or mixed with petrol. The combustion of ethanol produces carbon dioxide and water.
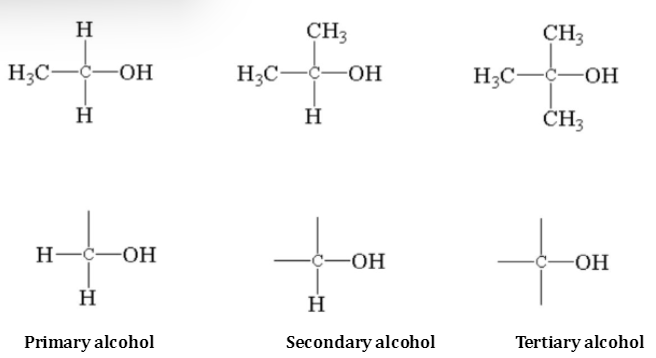
Primary alcohols - 1 carbon attached to C- OH group (like ethanol)
Secondary alcohols - 2 carbon attached to C- OH group
Tertiary alcohols - 3 carbon attached to C- OH group
The solubility of alcohols in water decreases the longer the chain gets...a longer chain means that more of the molecule becomes non-polar
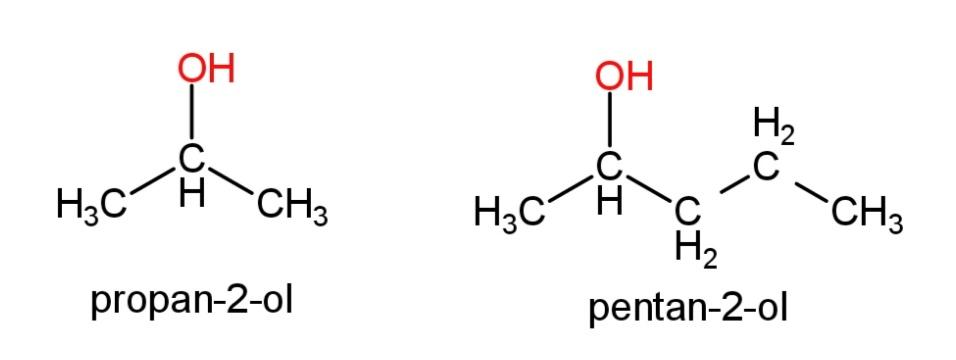
 Boiling point
Boiling point
Increases as size increases, High than alkanes, All alcohols are liquid at room temp, Alkanes / Alkenes are gases
High boiling point due to - OH group and hydrogen bonding = strengths the intermolecular bonds
Carboxylic acids
COOH group/ Carboxyl group. Suffix ‘oic acid’
The carboxyl group part of the molecule is polar.
The solubility of the carboxyl group in water decreases the longer the chain gets...a longer chain means that more of the molecule becomes non-polar
Replaces 3 H in a hydrocarbon , They are always positioned at the end of the molecule and they are always labelled as the first carbon
Electrons are drawn away from the hydrogen, allowing H+ ions to be donated in an acid-base reaction.
Able to create hydrogen bonds
OH is acidic
Found: Carboxylic acids weak organic acids. They are commonly found in nature, giving a sour taste to lemon juice and vinegar
![]()
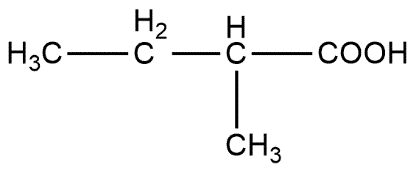
2 - methylbutanoic acid
Boiling point
Increases, as size increases
Higher than alcohols
Higher boiling point due to - COOH group and hydrogen bonding
Polymers
Large molecules made of repeating units called monomers.
High molar mass
How are they produced?
Through polymerization, where monomers are chemically bonded to form long chains or networks. Can involve using the same type of monomer or different types.
When different types of monomers are used, copolymers are created.
Catalysts are often needed for polymerisation reactions to occur efficiently.
Natural vs Synthetic polymers
Natural polymers
Most natural polymers are made of proteins or cellulose.
Plants are made of cellulose, and many contain another polymer called starch. Cellulose and starch consist of sugar monomers and are formed by condensation polymerisation.
Wool, silk, cotton, linen and rubber are all naturally occurring polymers.
Animals are made of proteins that are also condensation polymers.
Skin, organs, muscles, enzymes, hair and fingernails are all made of protein.
Synthetic polymers
Synthetic polymers are made from raw materials obtained from fossil fuels or from biomass.
Many synthetic polymers are called plastics in everyday life.
Synthetic polymers are commonly called plastics or synthetic fibres.
How are polymers made?
Polymers are usually made by either addition or condensation polymerisation.
Addition polymers are formed when an addition reaction causes monomers containing carbon-to-carbon double bonds (alkenes) to link together.
Condensation polymers are formed when molecules combine to form a larger molecule and a small molecule (such as H2O) is produced as a by-product.
What can plastics be classified into? And why?
Thermoplastics, Thermosetting plastics
Help determine the practical uses and recycling potential
Classified by how they are made and how they react to heat
Thermoplastics (thermosoftening plastics)
Made by reshaping, repeatedly melting and hardening by cooling.
Example: polystyrene, celluloid
Physical properties
Can be heated and reshaped, flexible and rigidity due to weak dispersion forces between the chains, can become malleable.
Will soften when heated, allowing it to be remolded and recycled.
Linear polymers
Are thermoplastic polymers that form long chains which become tangled due to their lengths.
What happens when linear polymers/thermoplastics are heated?
When heat is applied, molecules become free to move and it melts. The dispersion forces between the molecules breaks, it then cools down and the dispersion forces will reform giving a new structure ( strong covalent bonds are not affected by heat )
The length and degree of entanglement of the chains affects its properties.
Stronger forces and higher entanglement lead to a harder polymer.
Weak forces and lower entanglement results in greater flexibility.
Recyclable
MORE INFO IN NOTES
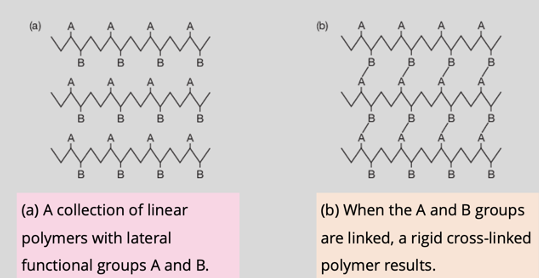
Thermosetting plastics/Thermosets
Thermosetting plastics are polymers that undergo irreversible crosslinking to form rigid and durable structures. Plastics that must be molded or shaped during their manufacture.
Crosslinked polymers
Have strong covalent bonds between the chains, making them rigid and strong, limits the movement
How to make crosslinked polymers?
Long chains with functional groups are produced and then crosslinked with heat or chemicals.
Example: Bakelite
What happens when thermosetting plastics are heated?
Very high temp covalent bonds are broken, it doesn’t melt, it burns, chars or decomposes.
Cannot be heated or reshaped again.
What are the impacts of cross-linking or bonding between the chains?
Affects the elasticity and rigidity of the polymer.
A small amount of cross linking produces an elastomer, which is relatively elastic.
A large amount produces a rigid polymer because atoms are strongly bonded in all three dimensions.
Cross link
Covalent bonds that connect neighbouring polymer chains.
Elastomers
Materials that can be stretched due to a small amount of cross- linking but return to their original shape when the stretching force is removed.
Examples: silicon rubber
Branching
High density
Linear polymers (without any branches) can get really close to each other and have stronger dispersion forces between polymers next to it.
—High density means it has low degree of branching so it is harder -crystalline sections
Higher melting point since chains are more closely packed, allowing for greater dispersion forces
Example, hard plastic like toys, cellulose (fiber), glycogen
LOWER DENSITY
If there is more branches attached to the linear polymers, all the polymer chains can't get closer to one another
More branching, more flexible -non-crystalline
Example, cling wrap
Plasticisers -Small little lumpy molecules in between the chains and it separates the chains, decreasing the dispersion forces -Makes polymers softer and more flexible -Lower density
Monomer
small molecules that are able to react to form long chains of repeatings units.
Additional polymerisation
The process in which monomers with a carbon-to-carbon double bond or triple bond is broken, allowing them to react and form long chains of repeating units.
Form when unsaturated monomer reacts.
During the polymerization process, one of the double bonds in the monomer is broken.
The electrons released from the broken bond are used to form a new bond with another monomer.
This linking process creates long chains of monomers, forming the polymer.
Small alkenes like ethene and propene are particularly suitable for this process.
How is polyethene made?
Ethene is the simplest monomer for addition polymerization, forming polyethene (polyethylene). Traditionally, ethene was derived from fossil fuels, but new methods now extract it from biomass. Under high pressure, ethene changes from a gas to a liquid. When heated in the presence of a catalyst (usually a small amount of oxygen), ethene molecules join in an addition reaction to form long polyethene chains. The length of these chains varies from 4000 to 20,000 carbon atoms depending on the temperature and pressure conditions.
Condensation polymerisation
The process in which a functional group of one monomer reacts with a functional group of another monomer.
During this reaction, a small molecule, often water, is eliminated.
Since each monomer has one more functional group remaining after the reaction, the process repeats, forming long polymer chains.
Remember to have open bonds at the end, include brackets and n (for number of monomers)
Example
Cellulose is a natural polymer found in plant cell walls, providing strength and stability.
It's abundant in plant materials like grains, vegetables, wood, paper, linen, and cotton.
Cellulose is the most common type of polysaccharide, meaning it's made of sugar monomers.
It's a linear polysaccharide formed by condensation polymerization of glucose monomers, with water released between each pair of monomers.
Hydrolysis
The chemical breakdown of a compound due to reaction with water. The ester bonds can be broken by hydrolysis and it returns to its original state, having the carboxyl and hydroxyl group again.
 Knowt
Knowt