BMS2045 2b Innate Immunity 2 2024
Theme Learning Outcomes
Innate Immune Response Features
Four Main Features: The innate immune response is characterized by its rapid action against pathogens, the presence of various defensive barriers, the ability to recognize pathogens through pattern recognition receptors (PRRs), and the inflammation process that helps recruit immune cells to sites of infection.
Cells and Mediators in Inflammation
Identifying Cells and Chemical/Protein Mediators: Key cells involved in inflammation include macrophages, neutrophils, mast cells, and eosinophils. Mediators include cytokines such as IL-1, IL-6, TNFα; chemokines; and other molecules that facilitate communication between cells and promote immune responses.
Function of Cytokines and Chemokines
Roles in Immunity: Cytokines are signaling proteins that modulate immune responses, including promoting inflammation and fever. Chemokines specifically attract immune cells to areas of infection or injury, playing a vital role in coordinating the immune response.
Mechanisms of Endocytosis
Comparative Mechanisms: Different endocytic mechanisms include phagocytosis, pinocytosis, and receptor-mediated endocytosis, with phagocytosis being crucial for engulfing large particles, such as pathogens.
Detailed Processes During Phagocytosis: Phagocytosis involves multiple stages: recognition, ingestion, digestion of the pathogen, and the final exocytosis of debris and presentation of antigens to adaptive immune cells.
Role of TLRs and PRRs
Involvement in Phagocytosis and Immune Cell Activation: Toll-like receptors (TLRs) and other PRRs recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), triggering phagocytosis and the activation of immune cells to respond effectively to infections.
Complement System Activation
Mechanisms and Functions: The complement system enhances the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promoting inflammation and attacking pathogen cell membranes.
Cell-Mediated Innate Immunity
Types of Cells and Their Killing Mechanisms: Specific cells like natural killer (NK) cells and cytotoxic T cells play a prominent role in targeting and eliminating infected or transformed cells through mechanisms such as granule exocytosis and cytotoxic cytokine secretion.
Innate Immunity
Four Main Types of Defensive Barriers/Features
Anatomical: Physical barriers, such as skin and mucous membranes.
Physiological/Chemical: This includes secretions like saliva, sweat, and stomach acid that hinder pathogen entry.
Phagocytic/Endocytic: Involves the action of phagocytes such as macrophages and neutrophils that ingest and destroy pathogens.
Inflammatory: The inflammatory response that increases blood flow to affected areas and recruits immune cells.
Phagocytosis Stages
Recognition: Phagocytes identify pathogens through receptors that bind PAMPs.
Ingestion: Following the binding of particles, phagocytes extend pseudopodia that engulf the pathogen in a membrane-bound phagosome.
Digestion: The phagosome fuses with lysosomes to form a phagolysosome, where pathogens are digested using both oxygen-dependent and independent mechanisms.
Exocytosis: The waste products of digestion are expelled, and some antigens may be presented on major histocompatibility complex (MHC) molecules for recognition by adaptive immune cells.
Ingestion Stage (2)
Mechanism of Ingestion: After receptor clustering, pseudopodia form, surrounding the particle and leading to its engulfment within a phagosome, which requires substantial energy and cytoskeletal rearrangement.
Fluid Dynamics in Phagocytosis (Page 6)
Role of Fluid Dynamics: Fluid forces assist in pushing pseudopodia around the particle, facilitating effective ingestion, which is critical for the phagocyte's activation during the recognition phase.
ER's Role in Phagocytosis (Page 7)
Contribution of Endoplasmic Reticulum: The endoplasmic reticulum contributes to the formation of phagosome membranes and supports the extension of pseudopodia, crucial for ingestion of pathogens.
Formation of Phagolysosome
Phagosome Formation and Fusion: After ingestion, lysosomes fuse with the phagosome to form a phagolysosome where the digestion process commences.
Digestion Stage (3)
Mechanisms of Digestion: Within the phagolysosome, both oxygen-dependent and oxygen-independent mechanisms are utilized to eliminate pathogens effectively.
Oxygen Independent Mechanisms
Processes: This includes acidification, action of lysozyme that digests bacterial cell walls, and other enzymes like lactoferrin, which inhibit microbial growth by binding iron essential for bacterial replication.
Acidification:
The phagolysosome acidifies at the same time as lysosome fusion enhancing theactivities of many enzymes and inhibiting growth of some pathogens
Lysozyme:
• Main enzyme within Lysosomes
• Mediates digestion of gram +ve bacterial cell walls
• ? Efficacy, works best in cooperation with other enzymes/systems
Other Enzymes
• Acid hydrolases: phosphatases, sulphatases, glycosidases, deoxyribonucleases• Lipases: eg phospholipase A2
• Neutral proteases: collagenases, elastase, cysteine proteases
Lactoferrin
• Binds to essential nutrients, inhibiting bacterial and fungal growth
Defensins• +ve charged polypeptides that bind -ve charged PAMPs e.g. LPS, LTA
• Electrostatically specific for microbial cells• Aggregate to form pores in cytoplasmic membranes
• Activate complement – classical pathway
• Most abundant protein in neutrophil granules
Cationic proteins
• Mainly found in Neutrophil granules, some in Eosinophils• HMW, Serine proteases, most active at alkaline pH
• Examples: elastase, cathepsin G, proteinase 3
• Damage microbial membranes & proteins
• Mostly anti-bacterial (cathepsin G also anti-fungal)
Tumour necrosis factor alpha (TNFa)
• Cytokine• Secreted (MFs mainly)
• Cytotoxic to tumour cells
Oxygen Dependent Mechanisms
Respiratory Burst Activation: A rapid release of reactive oxygen species occurs to damage cellular structures of ingested pathogens. Scavengers like catalase neutralize harmful byproducts to protect host cells while also being critical to resolving inflammation.
• Activated phagocytes produce Reactive Oxygen and ReactiveNitrogen Intermediates (ROI and RNI). These are molecules withunpaired electrons also known as ‘free radicals’
• Highly unstable and able to damage proteins, lipids, DNA and cellmembranes of microorganisms
• Oxygen radicals are short-lived but superoxide and H2O2 released intissues may persist and cause tissue damage
• RNIs also short-lived but may act with ROIs to give fullest effect
• Catalase, superoxide dismutase and glutathione are free radical
Reactive Oxygen Intermediates
• Generation of ROIs take place at low levels beneathplasma membrane in most cells = physiological activity
• Rapid increase in O2 consumption reflects a ‘burst’ oftargeted activity in phagosomes and phagolysosomes
• Involves cytoplasmic & membrane-associated enzymes:Nicotinamide Adenine Diphosphate (NADPH) -oxidase
• O2 converted to superoxide, peroxide or hydroxyl anionwith unpaired e-s which are highly reactive and damagemicrobial cell walls, DNA etc
Reactive Nitrogen Intermediates inducible Nitric oxide synthase (iNOS) activatedby microbial products and some cytokineseNOS and nNOS constitutively expressed and active in vasculature (endothelial, important forvascular tone) and neurones (NO can act asneurotransmitter) = normal physiological effects
Oxidises L-Argenine to yield L-Citrulline and NitricOxide (NO) with an unpaired electronl Within phagocytes = large ‘burst’ and LOTS of NOproduced with potent antimicrobial activity
Can combine with superoxide to be even morepotent
Phagocyte Mechanisms
Professional Phagocytes: Macrophages and neutrophils represent the primary phagocytes, employing different strategies for pathogen destruction. If pathogens evade direct intracellular killing, alternative processes such as autophagy or pyroptosis may occur.
• Most microbicidal mediators are produced within professional phagocytes (MC/MFs and Neus.)
• Difference is in location MC/MFs: lysosomes Neus: primary and secondary granules
• Neutrophils are more likely to kill ingested microorganisms due to higher respiratory burst and higher levels of defensins
• However, not all pathogens are killed by these mechanisms some escape to the cytoplasm however, these can be detected by cytoplasmic PRRs and undergo autophagy or succumb topyroptosis (initiated by the inflammasome formation)
Inflammation Overview
Immediate Defense Reaction: Inflammation represents a quick response to tissue damage and infection characterized by five main signs: redness, swelling, heat, pain, and loss of function.
Acute Phase Response: Occurs within the first 0-24 hours post-injury and aims to increase blood flow and permeability, facilitating leukocyte migration.
body’s immediate defence reaction to infection or damage
• 5 hallmarks: rubor (redness) et tumor (swelling) cum calor (heat) et dolor(pain), & loss of function• Acute phase response (0-24hours) differs from later inflammation andchronic inflammation
• Why needed?
• Events geared towards increasing blood flow,permeability of vasculature – allowing leukocyte migration to aid limiting the spreadof infection, tissue damage and to promote healing
• Involves large number of chemicals & cells acting together
Activation of Immune Cells During Inflammation
Mechanisms of Action: Inflammatory mediators enhance the expression of adhesion molecules and induce the release of neutrophils from the marrow, potentially leading to systemic responses such as fever.
Phagocytosis in Interaction with Inflammation: Phagocytes are vital in recognizing and disposing of pathogens, using receptors to identify PAMPs and DAMPs to trigger immune responses effectively.
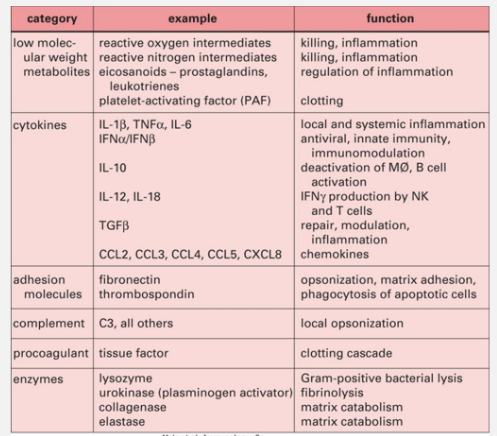
Inflammatory Mediators
Types of Mediators: Proteins, lipids, and chemicals released by various cells (especially mast cells and macrophages) contribute to inflammation. Examples include cytokines, chemokines, and leukotrienes, which mediate various aspects of the immune response.
Local effects:
MEDIATORS are released from activated tissue Cells (Mastcells/MFs)
Increase expression of adhesion moleculeson endothelial cells lining blood vessels &on leukocytes allowing them to attachto the vascular endothelium, facilitating their migration to sites of infection or injury.
Other mediators affect vasculartone and integrity of endotheliallayer allowing attached cells to pass into tissues
Systemic effects
Stimulate release of granulocytes &monocytes from Bone marrow
Hypothalamus – fever
Liver – increased Acute PhaseProtein production (CRP, SAA,MBL)
Migration of leukocytes to site of trauma/infection leading to increased phagocytosis and promotionof healingSiteLocal effects
At site of damage/infection, cells phagocytose toxins, damaged selfproteins/cells and pathogens - recognised through binding to cellreceptors for foreign molecules (PAMPs and DAMPs)
• Phagocytosis also stimulated by OPSONINS e.g. Antibody,Lectins/Acute Phase Proteins (such as C-reactive protein, MannoseBinding Lectin and Serum Amyloid A) and proteins of the complementactivation pathways (indirect recognition)
• Organism/toxin destroyed by anti-microbial proteins/peptides(AMPs) enzymes or by respiratory burst in granules/phagolysosomes,Neutrophil Extracellular Traps (NETs)
• The release of many mediators enhance these effects and affectlocal blood vessels to allow migration of more leukocytes to the site
Inflammatory mediators• Include a wide range of proteins, lipids and chemicals:
• Produced by a variety of cell types, but initially in inflammation by Mastcells, Basophils and MFs at sites of infection or damage
• Cells express receptors for these mediators
Prostaglandins, leukotrienes, thromboxanes, histamine…Cytokines (~58 including 37 interleukins): ‘cytokine’ suggestsmovement of cells and ‘interleukin’ suggestscommunication/messaging between white blood cells
Chemokines (~45): ‘chemokine’ suggests movement towards a chemical which is an ‘attractant’ (chemo-attractant)
• Additionally, plasma has 4 interconnected mediator producing systems:kinin, clotting, fibrinolytic and complement
Cytokines in Inflammation
Key Cytokines: Alarm cytokines like IL-1, IL-6, and TNFα enhance vascular permeability and promote fever and healing responses. Anti-inflammatory cytokines help regulate and resolve inflammatory processes.
• Many play a significant role in inflammation
• These include: Interleukin-1, 6, 8, 10, 12 (IL-1, IL-6 etc), Tumour necrosis factor a (TNF-a), Transforming growth factor β (TGF-β) and interferon γ(IFNg)
• IL-1, IL-6 and TNFα are ‘alarm’ cytokines for the acute phase (or earlyinflammation) causing local and systemic activation of fever, increased vascular permeability, production of Acute Phase Proteins and increased adhesion molecule expression. They are ‘pro-inflammatory’
‘Anti-inflammatory’ cytokines are able to down-regulate responses, includeIL-10 and transforming growth factor beta (TGF-β)
• IL-12 and IL-18 induce the differentiation of pro-inflammatory subset of Tcells• IL-8 is a potent chemokine for neutrophils
• IFNγ acts later in the acute phase contributing to chronic inflammation byrecruiting Mφs to sites of damage/infection
• Type I interferons (IFNa and b) have antiviral properties within infected cells
Chemokines and Chemoattractants
Role: Small proteins classified into families that guide migrating immune cells to their respective sites of action (chemotaxis), crucial for effective immune response.
• Chemoattractants
• Small proteins – two major families: CC and CXC contain 43 of the 45defined chemokines
• Defined by presence of 4 cysteine residues & sequence of amino acidsinvolving first two of these
• On binding specific receptors, stimulate migration & activation of rangeof cells towards the source (along a concentration gradient) =Chemotaxis
• e.g. IL-8, MCP-1 (Monocyte chemotactic protein 1)
Eicosanoids in Inflammation
Derived Compounds: Eicosanoids such as prostaglandins and leukotrienes play significant roles in mediating vascular changes, fever induction, and smooth muscle contraction during inflammation.
Other Inflammatory mediators Prostaglandins, Leukotrienes and Thromboxanes
• All are Eicosanoids = unsaturated fatty acids derived from arachidonicacid
• Prostaglandins made by enzymes including cyclooxygenases (COX-1,COX-2) – major target of ‘anti-inflammatory’ pain killers
• Described as having ‘hormone–like’ effects
• Act on vascular permeability and leukocyte migration to tissues
• PGE2 acts on hypothalamus to induce feverHistamine
• Acts to increase vascular permeability and smooth muscle contraction
Adhesion Molecules and Migration During Inflammation
Leukocyte Binding: Various adhesion molecules facilitate leukocyte attachment to the endothelium and aid in their migration into tissues, a process regulated by chemokines.
wide range of adhesion molecules, selectins and integrins promotebinding of leukocytes to endothelium
• Movement along or between is also regulated by release of chemokines causing chemotaxis
• Movement (or migration) of white blood cells or fluid from the lumen of blood vessels into tissues = extravasation
• Also important in lymphocyte homing
 Knowt
Knowt