Unit 9- Redox/Electrochemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here.
* Reminder: when an atom has a positive charge, there are less electrons due to them being negatively charged. Same for when atoms have negative charges, there it has more electrons.
* Any code blocks () will represent subscripts. Ex: Carbon Dioxide= CO2
Oxidation States/Numbers:
→ The charge of elements. (The ones on the upper right hand corner of the periodic table for each element)
- Since most elements have more than one oxidation state, you can calculate the exact ones for each element in a specific reaction or compound.
- An element could have an oxidation state not written in the periodic table.
Rule for calculating Oxidation states:
Oxygen is always 2-, with two exceptions
- H
2O2, O is 1- - OF
2, O is 2+
- H
Hydrogen and most group 1 ions are almost always 1+ (Can also be written as +)
Halogens (group 7 ions) will almost always be 1- (Can also be written as -)
Elements on their own (in a reaction) are always 0, unless a charge is written on the element symbol.
Diatomic elements are always 0.
In a compound the sum of the oxidation states must equal zero
- If it’s a polyatomic ion, the sum must equal the charge of the compound.
Examples of calculations:
- BeO
- Since O is always 2-, the oxidation state of Be must be 2+, due to the fact that the sum of the numbers should be zero in neutral compounds
Redox reactions:
→ Redox is short for “Oxidation Reduction”
Oxidation and Reduction:
- Oxidation → The lose of electrons by an atom (an atom becoming more positive)
- Reduction →The gaining of electrons by an atom (an atom becoming more negative)
- Think of it as the oxidation number is reducing, rather than the amount of electrons.
- A way to remember is LEO the lion says GER
- LEO stand for “Loosing electrons is Oxidation”
- GER stand for “Gaining electrons is Reduction”
The Reactions:
- In redox reactions one element is losing electrons (Oxidizing), while another is gaining those electrons (Reducing). These two thing happen simultaneously
- In these reaction the element which is oxidized (lost electrons), is known as the Reducing Agent.
- The Element which is reduced (gained electrons), is known as the Oxidizing Agent.
- The oxidation states/charges of elements will change from the reactants to the products.
For Example:
- Na + Cl → NaCl, is a redox reaction, since Na and Cl are both neutral on the reactants side, but have charges on the products.
- Na on the products side has a charge of 1+, and Cl has a charge of 1-.
- Na lost an electrons so it was oxidized (was the reducing agent)
- Cl gained an electron so it was reduced (was the oxidizing agent)
- If a reaction does not have both oxidation and reduction happening it is not a redox reaction.
Half Reactions:
→ Redox reactions must be balanced for BOTH mass and CHARGE. To do this we use half reactions to show both processes (Oxidation and Reduction).
- A redox reaction can be split in to two half reactions, one for the element being oxidized, and the other for the element being reducded.
- If there are any ions or elements where the charge doesn’t change, they are left out of these half reactions.
- These are known as spectator ions.
- In the half reaction we also show the electrons, represented by "e-”.
- Before writing half reaction make sure the mass is balanced (same amount of each element on both sides)
- Then when the half reaction are written, there should be the same amount of “e-” in both halves.
- If not then you need to manipulate them so they do.
- When the two half reaction are added together you can cancel out the “e-”, and you should be left with a balanced version of the original reaction
- Some reaction are already balanced for both.
Examples:
- Na + Cl → NaCl, same as above.
- The Oxidation half reaction would be: Na→ Na^1+ + e-
- The Reduction half reaction would be: Cl + e- → Cl^1-
- Charges are usually written as exponents/superscripts.
- Notice the e- lost by Na, is the same e- gained by Cl. (This reaction is already balanced)
- (Insert picture of 3. on pg 12)
Simultaneous Reactions and Table J:
- On Table J, metals that are higher up will be oxidized
- And the lower ones will be reduced.
- So if K and Ca were in a redox reaction, K should oxidize since it is higher up than Ca.
- If the metal that gets oxidized is lower, than the metal being reduced than the reaction will NOT be spontaneous. (There are scenarios where this could happen)
- If the metal that is oxidized is higher than the one being reduced, the reaction WILL be spontaneous.
Cells/Batteries:
→ There are two types of cells/Batteries: Galvanic/Voltaic cells, and Electrolytic cells. Though they work differently they do have some of the same parts. (They are both referred to as electrochemical cells)
- There are two electrodes in each battery. The electrons are where the electrons are oxidized and reduced.
- Anode → The electrode where oxidation will occur in the cell.
- Similar to an anion, because it is the negative end of a batterie.
- Cathode → The electrode where reduction will occur in the cell.
- Like a Cation, it is the positive end.
- You can remember these by using "An Ox”, and "Red Cat”
- An Ox, stands for Anode is Oxidation
- Red Cat, stands for Cathode is Reduction.
- The Anode and the Cathode are connected by a wire, where the electrons will flow through.
Voltaic/Galvanic Cells:
→ Both names mean the same thing.
- These cells produce energy
- The Anode and Cathode are separated, either in two separate containers filled with salt solutions, or by a membrane in the same container.
- There is still a wire for connection.
- Electrons will flow from the Anode to the Cathode.
- From the electrode which loses electrons to the one which gains electrons.
- A salt bridge also connects the two solutions in the beakers. This helps maintain equilibrium by moving the icons around.
- The ions will flow the opposite way as the electrons. from the cathode side to the anode side.
- As the cell goes through Oxidation and Reduction the Anode will lose mass and the Cathode will gain mass.
- This is because the Anode loses atoms to the solution as they become ions from losing their electrons. (They fall off of the Anode)
- The Cathode gains those electrons, which the ions in the solution will accept and they will then become neutral atoms and attach to the Cathode.
- It converts chemical energy to electrical energy.
Example: (Picture)
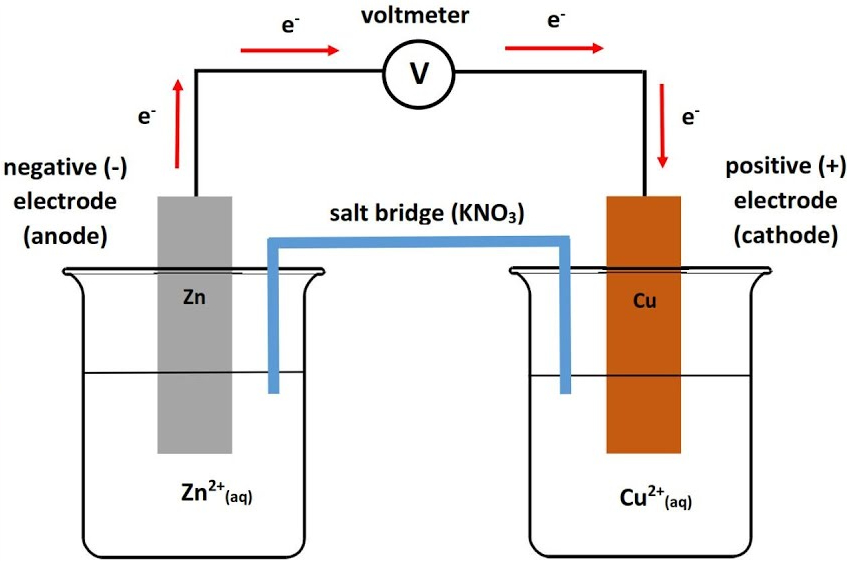
- Zn is the anode because it is higher up on Table J, than Cu, so it will oxidize.
- The electrons are flowing from Zn (anode) to the Cu (Cathode)
- The salt bridge is carrying ions from the Cu side to the Zn side.
- Over time the anode will lose Zn atoms as they become ions, and the cathode will gain Cu atoms as they become neutral.
Electrolytic Cells:
- These cells require external energy to work. (Usually a battery)
- This is because it is forcing a spontaneous reaction to happen.
- The Anode and Cathode are switched. So the one being oxidized is the one which is usually reduced in a Galvanic cell.
- They convert electrical energy into chemical energy
- There is no salt bridge since the entire cell is in one container.
- These cells are usually used to cat items so the cathode is usually an item like a key or some type of cutlery
- This process is known as electroplating.
Example: (Picture)
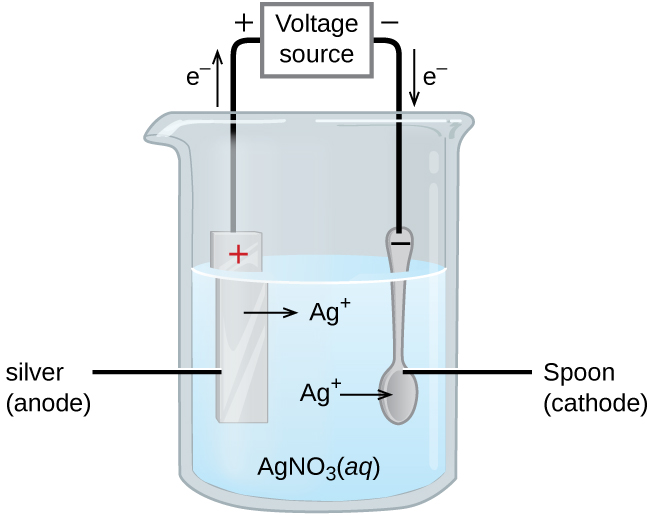
- The Silver anode is losing electrons and those electrons are being transferred to the spoon.
- The spoon accepts them and then the Ag+ ions in the solution take the electrons and attach to the spoon.
- This will plate the spoon in silver. Increasing the mass of the spoon.
- The voltage source gives electricity to force the nonspontaneous transfer of electrons